Ann Clin Microbiol 2024;27:69-79. Molecular diagnosis of parasitic diseases in Korea
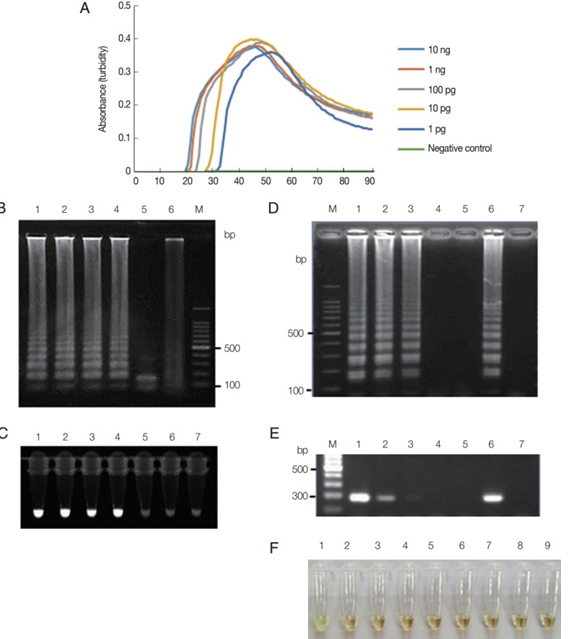
Fig. 1. Functionality of Trichomonas vaginalis actin LAMP assays. (A) LAMP on 10-fold serial dilutions of T. vaginalis genomic DNA (10 ng to 1 pg per reaction) monitored by measuring absorbance. Distilled water was used as a negative control. (B) LAMP products were visualized by gel electrophoresis. Lane 1, 1 ng; Lane 2, 100 pg; Lane 3, 10 pg; Lane 4, 1 pg of T. vaginalis genomic DNA; Lane 5, LAMP product after HindIII digestion; Lane 6, distilled water; andLane M, 100-bp DNA marker. (C) LAMP products were visualized under UV light using Loopamp fluorescent detection reagent. Lane 1, 1 ng; Lane 2, 100 pg; Lane 3, 10 pg; Lane 4, 1 pg; Lane 5, 100 fg; Lane 6, 10 fg of T. vaginalis genomic DNA; and Lane 7, distilled water. (D and E) T. vaginalis at a density of 1×102 parasites/μL was serially diluted and tested using LAMP assay (D) and PCR (E) with F3 and B3 primers (Table 1). Lane M, 100-bp DNA marker; Lane 1, 100; Lane 2, 10; Lane 3, 1; Lane 4, 0.1; Lane 5, 0.01 parasite (s) per reaction; Lane 6, positive control, 100 pg of plasmid DNA containing LAMP targeting regions of actin gene; and Lane 7, distilled water. (F) Specificity of LAMP primers for detection of T. vaginalis assessed using template DNA from other microbial species. Lane 1, T. vaginalis; Lane 2, Candida albicans; Lane 3, Chlamydia trachomatis; Lane 4, Neisseria gonorrhoeae; Lane 5, Cryptosporidium parvum; Lane 6, Entamoeba histolytica; Lane 7, Giardia lamblia; Lane 8, Escherichia coli; and Lane 9, human genomic DNA. LAMP products were visualized via color change that was also observable by the naked eye under normal visible light reproduced under the CC-BY-NC license from the original article [19].