Abstract
Background: Inconclusive SARS-CoV-2 real-time reverse transcription-PCR (rRT-PCR) test results, which are positive for one or more target genes but not all, are problematic in clinical laboratories. In this study, we aimed to investigate the cause and clinical relevance of such inconclusive results.Methods: rRT-PCR was performed using the Allplex 2019-nCoV assay kit (Seegene Inc., Korea) targeting the following three genes: E, RdRp, and N. For all inconclusive test results reported from March to June 2020, the frequency per kit, lot number, specimen type, cycle threshold (Ct) and peak values of the amplification curves, positive target genes, and results of repeated or consecutive tests were analyzed.Results: A total of 43,268 tests were conducted, of which 93 (0.21%) were inconclusive—49 from 11 coronavirus disease (COVID-19) patients and 44 from non-COVID-19 patients. In COVID-19 patients, the results were inconclusive 11.9 ± 4.7 days after diagnosis and were negative 8.8 ± 5.5 days after the inconclusive results were reported. However, in non-COVID-19 patients, they were all negative upon retest and 81.8% of them were identified to have yielded in 2 out of 8 lots. The most frequently positive target genes were N (55.4%) in COVID-19 and RdRp (61.2%) in non-COVID-19 patients, respectively. No difference was observed in the Ct or peak values of the amplification curves for inconclusive samples between COVID-19 and non-COVID-19 cases.Conclusion: Inconclusive test results should be reported neither positive nor negative. Such results can be reported as inconclusive without retesting in COVID-19 patients; however, they should certainly be confirmed by a retest in non-COVID-19 patients or newly diagnosed cases.
Keywords
Coronavirus disease, Inconclusive results, Polymerase chain reaction, Severe acute respiratory syndrome coronavirus 2
Figures & Tables
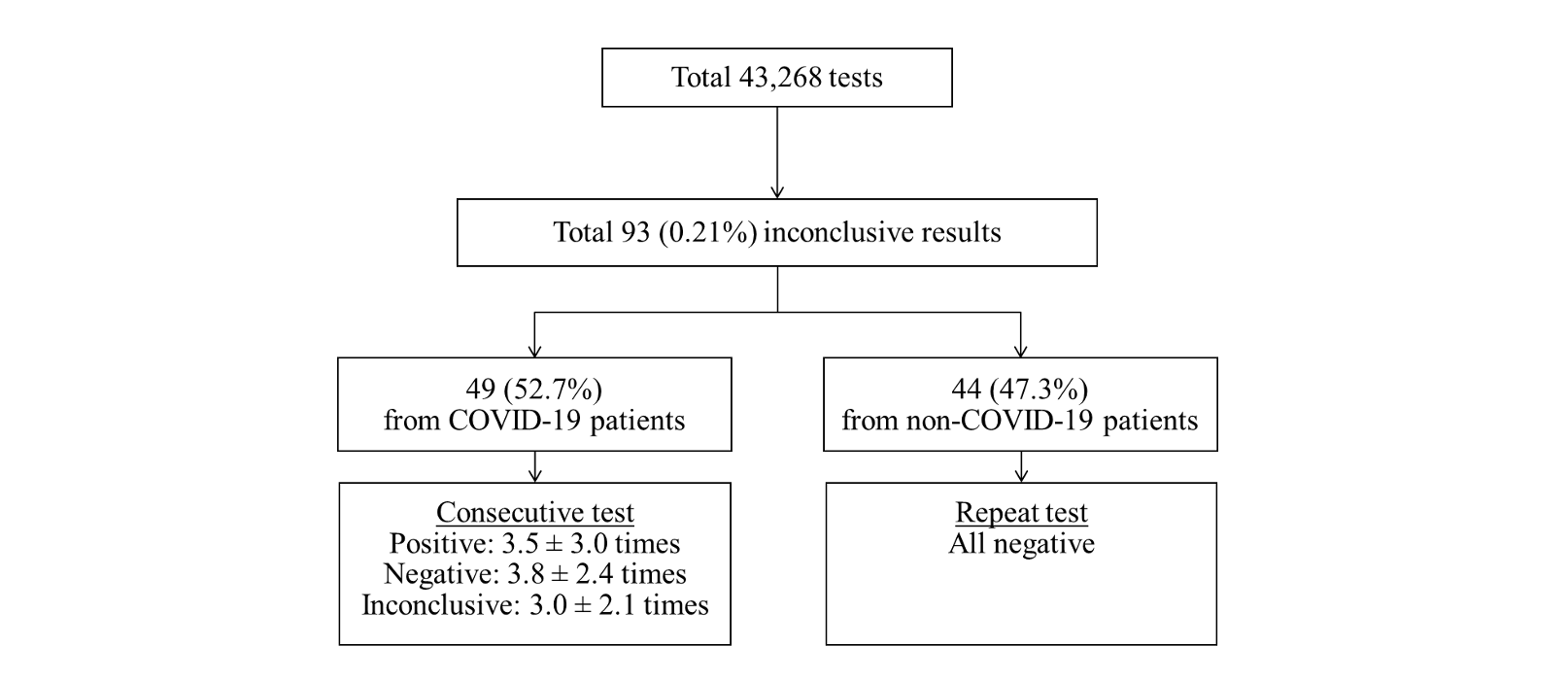
Fig. 1. Classification of the inconclusive results according to COVID-19 diagnosis and retest or consecutive test results.
Figures & Tables
Table 1. Summary of rRT-PCR test producing inconclusive results from 11 COVID-19 patients
| Patient | Anti-COVID-19 treatment | Days from diagnosis to | Ct values of the initial inconclusive result | Days and number of consecutive test results from initial inconclusive results to negative conversion | ||||||
| Admission | Initial inconclusive result | E | RdRp | N | Days | Pos | Inc | Neg | ||
| 1 | N/A | 0 | 9 | N/D | 35.35 | 36.5 | 21 | 10 | 5 | 3 |
| 2 | Lopinavir/ritonavir | 6 | 6 | 35.4 | 35.28 | N/D | 11 | 2 | 5 | 8 |
| 3 | N/A | 0 | 21 | N/D | N/D | 38.77 | 11 | 3 | 3 | 1 |
| 4 | ECMO, steroid | 6 | 15 | 32.99 | N/D | 37.74 | 10 | 6 | 6 | 6 |
| 5 | Lopinavir/ritonavir, Camostat mesylate, HCQ | 2 | 12 | 35.71 | N/D | N/D | 4 | 0 | 3 | 2 |
| 6 | Camostat mesylate | 0 | 15 | N/D | 38.7 | 37.35 | 5 | 1 | 2 | 2 |
| 7 | HCQ | 0 | 5 | 33.65 | N/D | 34.56 | N/A* | N/A* | N/A* | N/A* |
| 8 | N/A | 68 | 76 | N/D | N/D | 38.69 | 2 | 0 | 0 | 1 |
| 9 | Plasma treatment | 0 | 14 | 37.12 | 38.22 | N/D | 3 | 3 | 0 | 2 |
| 10 | Lopinavir/ritonavir, HCQ, plasma treatment | 1 | 10 | N/D | 38.95 | N/D | 5 | 3 | 0 | 6 |
| 11 | N/A | 0 | 0† | N/D | N/D | 36.91 | N/A† | N/A† | N/A† | N/A† |


