Abstract
16S rRNA gene-targeted next-generation sequencing (NGS) can detect microorganisms in a comprehensive reference database. To date, NGS has been successfully applied to samples such as urine, blood, and synovial fluid. However, there is no data for continuous ambulatory peritoneal dialysis (CAPD) fluid. The purpose of this study was to evaluate the clinical usefulness of microbiome analysis of CAPD fluids for the diagnosis of CAPD peritonitis.We included 21 patients with high suspicion of CAPD peritonitis. Routine CAPD fluid culture was performed using a pellet of 50 mL CAPD fluid onto the chocolate and blood agar for two days, and thioglycollate broth for one week. 16S rRNA gene-targeted NGS of pellets, stored at -70°C was performed with MiSeq (Illumina, USA). Many colonized or pathogenic bacteria were detected from CAPD fluids using NGS and the microbiomes were composed of 1 to 29 genera with a cut-off 1.0. Compared to the culture results, NGS detected the same pathogens in 6 of 18 valid results (three samples failed with low read count). Additionally, using NGS, anaerobes such as Bacteroides spp. and
Figures & Tables
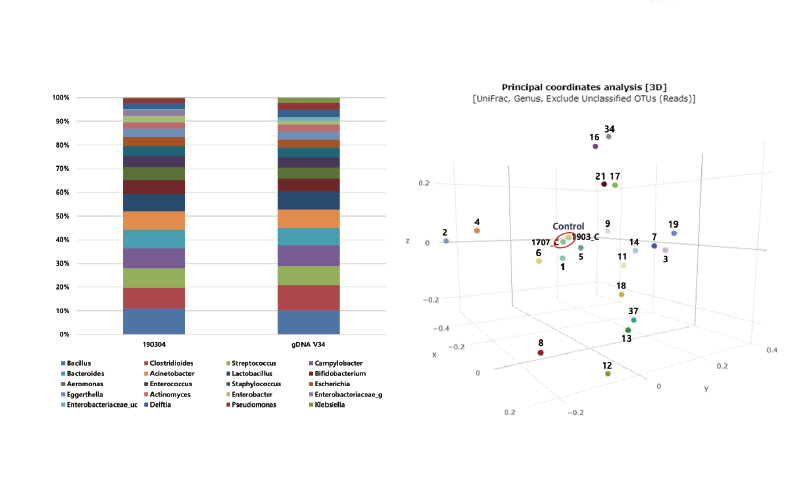
Fig. 1. 1. Composition of gDNA Mock V34 (1707_C) and MiSeq QC (1903_C) (A), the principal coordinates analysis with CAPD fluid samples with QC controls in each run (B).Abbreviation: CAPD, continuous ambulatory peritoneal dialysis; QC, quality control; OTU, operational taxonomic unit


