False Positive Cases in Automated Blood Culture Systems Due to Hyperleukocytosis
Department of Laboratory Medicine, Keimyung University School of Medicine, Daegu, Korea
ABSTRACT
Automated blood culture systems are widely used in clinical microbiology laboratories to minimize the workload of laboratory personnel and permit fast turnaround times. However, sometimes false positive signals occur due to leukocytosis, presence of fastidious bacteria, or unexplained causes. We experienced false positive signal in a patient with hyperleukocytosis for the first time since the automated blood culture system was introduced in our hospital over 20 years ago. We present two case reports with literature review and describe the procedure for dealing with false-positive cases in our hospital.
Keywords
Automated blood culture systems, False positive, Hyperleukocytosis
INTRODUCTION
Bloodstream infections (BSIs) are major health concerns and can cause serious problems as they are associated with high mortality and morbidity rates. Blood culture is the gold standard method for the detection of BSIs. The automated blood culture systems continuously monitor blood culture bottles and detect microbial growth based on internal algorithms. Rapid detection of BSIs is important in achieving better patient outcomes. However, false positive signals occasionally occur due to leukocytosis, the presence of fastidious bacteria, or unexplained causes. We have experienced false positive signals in patients with hyperleukocytosis and present cases with the literature reviews and introduce the procedures of false-positive cases within our hospital
CASE REPORT
A 27-years old woman visited Keimyung University Dongsan Hospital with a high fever (39.5℃), leukocytosis, and splenomegaly in June 2022. Her initial complete blood count (CBC) revealed a hemoglobin (Hb) of 7.8 g/dL (normal range: 11-15 g/dL) with a reticulocyte count of 1.74%, a total white blood cell (WBC) count of 500.0×109/L (blasts, 10%), and a platelet count of 612×109/L. Blood urea nitrogen was 21 mg/dL (normal range: 6-20 mg/dL), and creatinine was 1.59 mg/dL (normal range: 0.5-0.9 mg/dL). Bone marrow (BM) studies with cytogenetic and polymerase chain reaction (PCR) tests for BCR-ABL1 were tested. BM aspiration revealed markedly hypercellular marrow particles with markedly increased myeloid lineage. The blasts were about 6% of all nucleated elements with increased basophils and eosinophils. BCR-ABL1 major gene rearrangement was confirmed with PCR. Chromosomal analysis showed 46, XX, t(9;22)(q34;q11). Therefore, the patient was diagnosed with chronic myeloid leukemia (CML). She was then treated with dasatinib and her WBC decreased. Blood culture tests were done on the day of admission due to the high fever. The next day, the automated blood culture system flagged a positive signal. However, Gram stain of culture broth revealed no bacteria but many WBCs. Moreover, an abrupt increase of peak was noticed in the reading graph within three hours of incubation in BacT/Alert Virtuo (bioMérieux, Durham, NC, USA) system (Fig. 1).
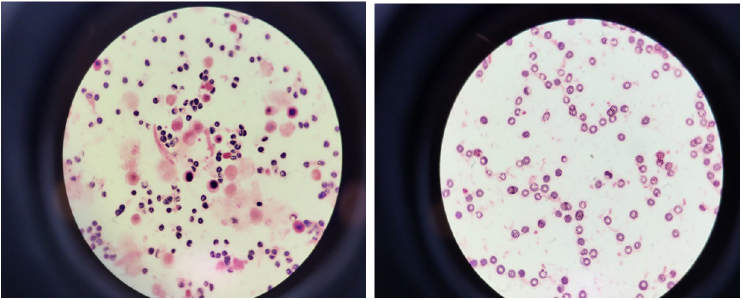
Fig. 1. Gram stain of initial blood culture of positive signal showed no bacteria, but with many WBCs (×1,000, left-first case; right-second case). WBC, white blood cell.
The blood culture was resumed until five days in the system along with the incubation of blood agar plate in the incubator at 35℃ under 5% CO2. However, we observed no further changes of peak, no bacteria in the Gram stain, and negative on blood agar plate (Fig. 2). The patient progressed well and was discharged for regular outpatient follow-up. Her laboratory results resumed to normal with Hb of 11.1 g/dL, WBC of 9.1×109/L, and platelet of 131×109/L after two months of discharge from the hospital.
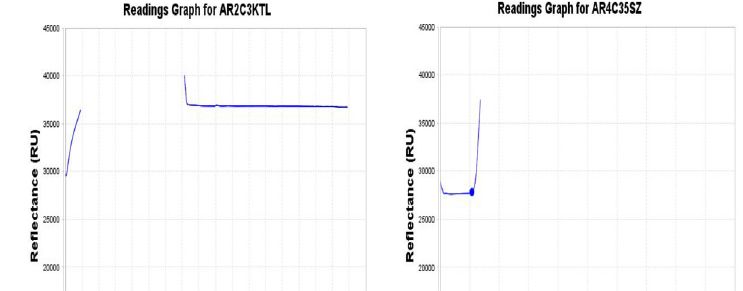
Fig. 2. Reading graphs of blood culture bottle of the first patient (A) showed positive flag within less than a day after starting to incubate in the automated blood culture system. Additional incubation of blood cultures revealed no change of peaks in reading graphs until 5 days. (B) Normal positive flag within a day showed abrupt peak at 13 hours after incubation.
The second case was 84 years-old man admitted to our hospital due to melena and leg pain with ecchymosis in October 2022. His peripheral blood smear and CBC showed a markedly increased WBC count of 440.38×109/L with 4% blasts. He was then diagnosed with CML. However, his general condition deteriorated, with low blood pressure, acute kidney injury, and loss of consciousness. Therefore, no further tests could be done except for blood cultures. He expired on the second day of admission. His blood culture tests were positive within four hours of incubation in the BacT/Alert Virtuo (bioMérieux) system. However, we observed no bacteria in the Gram stain and no bacterial growth on the agar (Fig. 1).
This study was approved by the Institutional Review Board of Dongsan Medical Center (IRB-2023-03-019).
DISCUSSION
In clinical microbiology laboratories, blood cultures are important to find out the pathogens causing bacteremia or fungemia [1]. The blood culture using automated blood culture systems with continuous monitoring method provides results within five days. Ruiz-Giardin et al. [2] stated that the median time of growth detection was 12.72 hours in the bacteremia patients. However, Qian et al. [3] reported that the time to positivity was significantly shorter in false-positive cases, mostly less than a day (< 24 hours) than in true-positive bacteremia cases, all more than a day (> 24 hours). In our study, two cases both showed positive signals in less than 6 hours. There is a slight time difference between two studies with ours, but it should be aware that the earlier signal more chance of being false signal. Therefore, distinguishing between true and false bacteremia is crucial for early treatment after right microorganism identification in a patient with sepsis.
Blood cultures detected as positive by the automated blood culture system but no bacteria in Gram stain by microscopy and no growth on subculture are considered as false-positive. Hyperleukocytosis or the presence of fastidious bacteria have been considered frequent causes of false-positives, but unexplained cases remain to be explored [4-6]. Ziegler et al. [7] found out that false-positive rates of blood cultures using blood culture systems were between 1.4% and 6.2%. BacT/Alert Virtuo (bioMérieux) system based on colorimetric detection by pH sensors of produced CO2 by growing microorganisms. Positive signals appeared in automated blood culture systems due to hyperleukocytosis may contribute to false-positive culture results as the metabolism of leukocytes also could generate CO2 and resulted in increases of CO2 [7]. Direct identification of culture positive bottles by molecular methods is applicable and could be considered to differentiate the true and false positives. However, it needs extra work and expense in routine microbiology laboratories [3]. Therefore, most laboratories do not use additional molecular tests for identification as in our laboratory.
We considered the first case the very first experience in the clinical microbiology laboratory of our hospital. Still we might have missed the cases and were unaware of the abnormal findings. Therefore, we recommend specific procedures to deal with the suspicion of false-positive cases. Using an automated blood culture system, we often find no bacteria in Gram stain by microscopy when positive signal flagged bottles were processed. First, we recommend checking the reading graph to see whether a peak appeared within a few hours of incubation. If a peak was observed, secondly, the laboratory test results should be reviewed for leukocytosis (neutrophilia) and the presence of other signs to consider true bacteremia. Third, additional blood culture incubation should be sustained until five days with subculture on the agar under CO2 to enhance the growth of fastidious bacteria. Finally, Gram stain examination on the blood culture media and subculture on an agar plate is recommended to exclude the false negative.
요약
자동화 혈액배양 시스템은 혈액배양 검사의 소요시간을 단축하고 검사실 업무를 줄일 수 있기에 임상미생물 검사실에서 널리 사용되고 있다. 그러나, 백혈구증가증, 까다로운 세균의 증식 또는 아직 밝혀지지 않은 이유로 인해 위양성으로 검출되기도 한다. 자동화 혈액배양 시스템을 사용한지 20년 동안 처음으로 백혈구증가증에 의한 위양성을 경험하였다. 이에 저자들은 위양성 시그널을 확인한 경험 2례를, 문헌고찰과 함께 위양성을 인지하는 방법과 처리 절차를 포함하여 보고하는 바이다.
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this article were reported.
REFERENCES
1. Opota O, Croxatto A, Prod’hom G, Greub G. Blood culture-based diagnosis of bacteraemia:state of the art. Clin Microbiol Infect 2015;21:313-22. 

2. Ruiz-Giardin JM, Martin-Diaz RM, Jaqueti-Aroca J, Garcia-Arata I, Martin-Lopez JVS, Buitrago MS. Diagnosis of bacteraemia and growth times. Int J Infect Dis 2015;41:6-10. 

3. Qian Q, Tang YW, Kolbert CP, Torgerson CA, Hughes JG, Vetter EA, et al. Direct identification of bacteria from positive blood cultures by amplification and sequencing of the 16s rRNA gene: evaluation of BACTEC 9240 instrument true-positive and false-positive results. J Clin Microbiol 2001;39:3578-82. 


4. Khan M, Siddiqi R, Konopleva M, Bhatti MM, Benton CB. Increased peripheral leukemia blasts leading to false-positive blood culture. Blood Cells Mol Dis 2017;64:8-9. 

5. Ebihara Y, Kobayashi K, Watanabe N, Taji Y, Maeda T, Takahashi N, et al. False-positive blood culture results in patients with hematologic malignancies. J Infect Chemother 2019;25:404-6. 

6. Martinez RM, Martinez R, Partal Y, Casas J, Llosa J, Almagro M. An infrequent cause of false-positive blood cultures. Clin Microbiol Newsl 1993;15:7-8. 
7. Ziegler R, Johnscher I, Martus P, Lenhardt D, Just HM. Controlled clinical laboratory comparison of two supplemented aerobic and anaerobic media used in automated blood culture systems to detect bloodstream infections. J Clin Microbiol 1998;36:657–61. 


