Department of Pediatrics, Ajou University School of Medicine, Ajou University Hospital, Suwon, Korea
Correspondence to Hyun Joo Jung, E-mail: free1109@ajou.ac.kr
This article is a secondary publication of the original work published in Korean in the Korean Journal of Healthcare-associated Infection Control and Prevention (2024;29(2):110–115, https://doi.org/10.14192/kjicp.2024.29.2.110). This English version has been translated and published in Annals of Clinical Microbiology with the permission of the editors of both the Korean Journal of Healthcare-associated Infection Control and Prevention and the Annals of Clinical Microbiology. This secondary publication complies with the conditions set forth in the “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals” by the International Committee of Medical Journal Editors (ICMJE), and serves to enhance accessibility and value by making the content available to a broader international readership through English translation.
Ann Clin Microbiol 2025;28(2):8. https://doi.org/10.5145/ACM.2025.28.2.2
Received on 15 May 2025, Revised on 28 May 2025, Accepted on 30 May 2025, Published on 4 June 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Effective healthcare policies, such as vaccination, decreased the global prevalence of infectious diseases, such as pertussis. However, these diseases have recently re-emerged, posing a serious public health threat. This article discusses the recent pertussis outbreak in Korea, outlining its clinical symptoms and highlighting the relevant diagnostic tools and management strategies to prevent its re-emergence. Incidence of pertussis in South Korea has been increasing since 2015, with over 30,000 cases reported until November 1, 2024, marking the highest number of cases recorded since the 2000s. Although pertussis is fatal in infants, it can be prevented via maternal vaccination. However, in recent years, pertussis vaccination rate during pregnancy has remained at approximately 60%, which is insufficient to prevent neonatal pertussis. Notably, vaccination rates among adolescents and adults are even lower than those among children, leading to the rapid increase in pertussis infection in the post-adolescent and vulnerable populations. Therefore, effective strategies to promote the vaccination of adults, especially pregnant women, are necessary to prevent and control such re-emerging infectious diseases.
Pertussis, Vaccines, Whooping cough
Re-emerging infectious diseases are conditions that have significantly declined, often owing to public health interventions such as vaccination, but have resurged and now pose renewed medical and public health challenges [1]. Diseases such as measles, varicella (chickenpox), and pertussis (whooping cough), which are relatively well-controlled through vaccination, have recently shown an increasing incidence, raising both domestic and international concerns [2]. In South Korea, where classical pertussis cases are rare owing to widespread vaccination, many healthcare professionals may lack clinical experience with the disease. As a result, it may be difficult to effectively diagnose and manage pertussis in patients presenting with severe coughing. This article reviews the recent domestic trends in the incidence of pertussis and summarizes the essential clinical features, diagnostic methods, and preventive strategies. The aim is to support better recognition and management of pertussis and contribute to mitigating its re-emergence.
Pertussis, also known as whooping cough, is an acute respiratory illness caused by Bordetella pertussis. The causative organism is a gram-negative coccobacillus, with humans as its only known reservoir [3–5]. Pertussis is one of the most highly contagious diseases among all pediatric infections, with an attack rate of 80%–100% in susceptible individuals. It is especially prevalent in children under 5 years of age [4–6]. The younger the age group, the higher the mortality rate, with the highest rates observed in infants under 1 year of age [5,7,8].
In South Korea, pertussis is classified as a notifiable class 2 infectious disease and is subjected to nationwide surveillance. In countries with active vaccination programs, including South Korea, the incidence of pertussis has significantly declined [5,9]. However, recently, an increase in the number of pertussis cases has been observed in several countries, particularly among adolescents and adults with waning immunity. This resurgence has led to a subsequent increase in pertussis cases among young infants who were either unvaccinated or incompletely vaccinated. For instance, in the United States, the annual number of pertussis cases in infants, which remained below 50 before 2000, exceeded 100 in the 2010s [8–10].
According to nationwide surveillance data provided by the Korea Disease Control and Prevention Agency through its Infectious Disease Portal, as of November 2024, the incidence of pertussis in South Korea has reached its highest level since 2000, including both confirmed and clinically suspected cases (Fig. 1). Prior to 2023, pertussis cases showed an increasing trend from 2015, with annual cases rising to 230 in 2012, 205 in 2015, 318 in 2017, and peaking at 980 in 2018. However, the number of reported cases decreased during the coronavirus disease 2019 pandemic. This trend was reversed in 2023, with 292 reported cases, and by November 1, 2024, the number of cases surpassed 30,000.
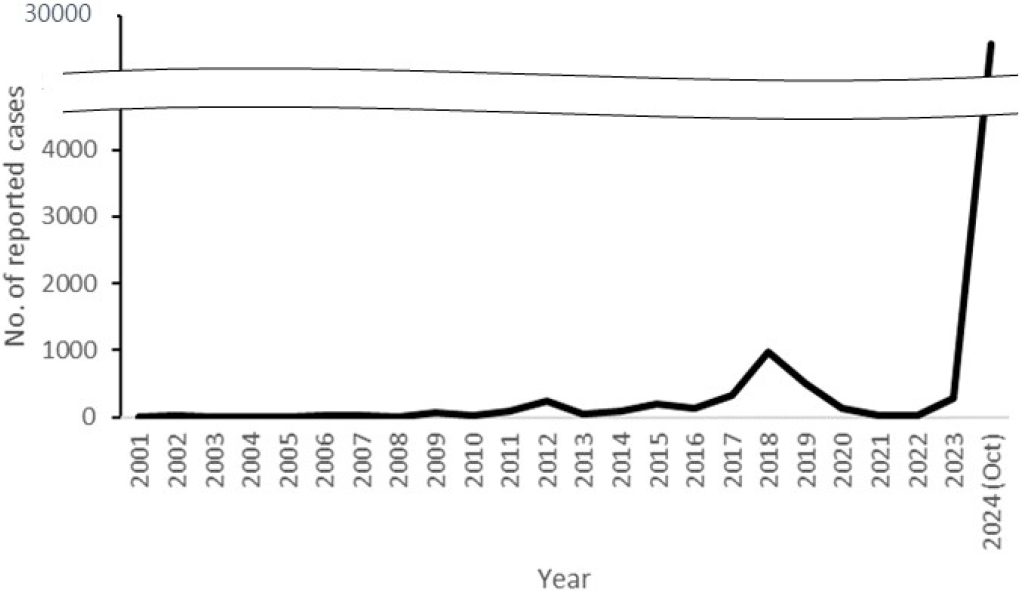
Fig. 1. Annual numbers of pertussis cases, Korea, 2001-2024 (Jan-Oct).
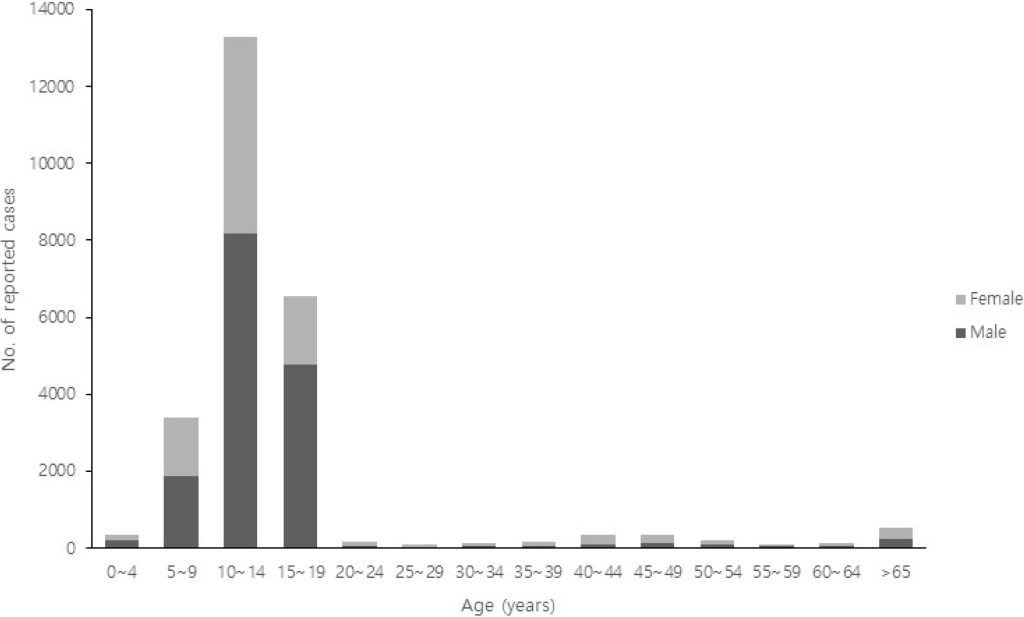
Regionally, cases have been more concentrated in densely populated and highly mobile areas, such as Seoul, Gyeonggi, and Incheon, although the outbreak has affected regions nationwide (Fig. 2). Adolescents were the most affected in terms of age distribution, with individuals aged 10–14 years accounting for 51.3% (13,283 cases) and those aged 15–19 years accounting for 25.4% (6,563 cases). Notably, cases have also been reported in infants under 1 year of age, highlighting the need for heightened vigilance in this vulnerable population (Fig. 3).
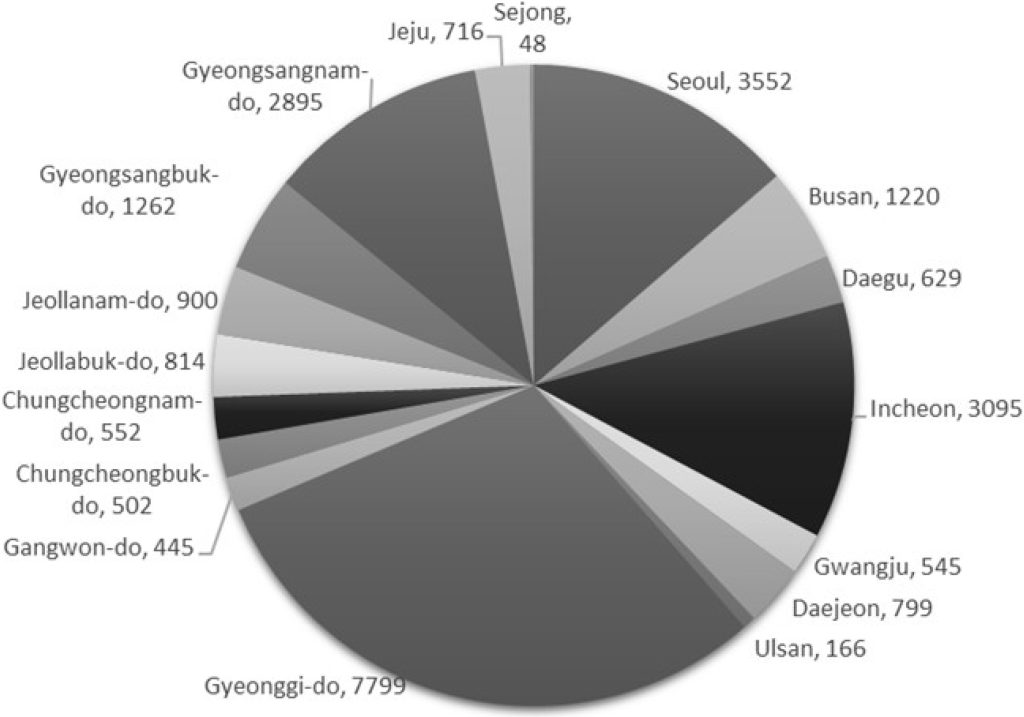
Fig. 2. Numbers of cases of pertussis by regions, Korea, January-October 2024.
Pertussis is transmitted primarily through direct contact or via respiratory droplets expelled during coughing [4–6]. Its transmissibility is extremely high, and adults infected with B. pertussis, especially those who do not exhibit classic symptoms, can serve as major sources of infection. In particular, household transmission and spread from adolescents and adults with prolonged, mild coughs to infants and young children are persistent public health concerns [11–13]. The period of highest infectivity is during the first two weeks after the onset of coughing. Early initiation of antibiotic therapy can reduce both the duration of illness and the period of contagiousness [4,14–16].
This stage lasts 1–2 weeks and is characterized by symptoms resembling a mild upper respiratory tract infection, including rhinorrhea, conjunctivitis, tearing, mild cough, and low-grade fever. This is the most contagious period of the illness.
Characteristic symptoms appear approximately 2 weeks after the onset of cough. The patient experiences repeated bouts of short, forceful expiratory coughs followed by a high-pitched inspiratory “whoop.” However, infants under 6 months of age may have a shorter catarrhal phase and may not exhibit the typical whooping sound. Instead, they may present with atypical symptoms, such as vomiting, respiratory distress, or apnea after coughing episodes, which require careful attention.
During the final stage, the intensity and frequency of coughing gradually decreases. Clinical manifestations vary depending on age, vaccination status, and the presence of passive maternal antibodies. In adolescents and adults, particularly those previously vaccinated, the disease may present with only a mild, persistent cough lasting more than a week without classic signs, making the diagnosis more challenging [19–21].
Complications are most severe in infants under 6 months of age. The case fatality rate in this group can reach 1%, and > 80% of pertussis-related deaths occur in children < 1 year of age, particularly among those younger than 2 months. Serious complications include apnea, pneumonia, seizures, and encephalopathy. During the paroxysmal stage, pressure-related complications, such as pneumothorax, subcutaneous emphysema, intracranial hemorrhage, rib fractures, and rectal prolapse, may occur. Vaccination significantly reduces the incidence and severity of these complications [3,17,18].
Key factors for diagnosing pertussis include a history of exposure, the presence of characteristic symptoms such as paroxysmal coughing lasting > 14 days, and features such as whooping or post-tussive vomiting [4–6].
Laboratory findings reveal leukocytosis with absolute lymphocytosis during the late catarrhagic and early paroxysmal stages. Chest radiographs may reveal mild bilateral perihilar infiltrates, pulmonary edema, or areas of atelectasis. However, these findings are nonspecific and not diagnostic of pertussis, indicating that blood tests and radiographic imaging should not be used as definitive diagnostic tools.
The most accurate method for confirming pertussis is bacterial culture of nasopharyngeal aspirates, swabs, or sputum samples. Culturing was most successful during the catarrhal and early paroxysmal stages. However, prior antibiotic use or vaccination can significantly lower the yield and the culture process requires considerable time, which may delay diagnosis.
Polymerase chain reaction (PCR) testing has recently gained recognition as a diagnostic method comparable to culture and is recommended by both the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention [4,18,22]. However, owing to the possibility of false positives and variability in test performance, PCR results should always be interpreted in conjunction with the clinical presentation and epidemiological context.
Clinically, pertussis must be differentiated from pertussis-like syndromes caused by infections such as adenovirus and Bordetella parapertussis as well as lower respiratory tract infections caused by Haemophilus influenzae, Mycoplasma pneumoniae, and Chlamydia. Other differential diagnoses include tuberculosis and foreign body aspiration [5,6,18].
The effective management of pertussis requires a combination of antimicrobial therapy and supportive care [4,18,23]. Antibiotic treatment not only alleviates symptoms but also plays a crucial role in preventing secondary transmission. Antibiotics should be administered not only to confirmed cases but also to suspected cases in high-risk individuals for complications, as well as to all close contacts of confirmed cases. The recommended types and dosages of antibiotics are listed in Table 1.
Table 1. Antibiotic regimens for treatment and prophylaxis of pertussis
| Age | Macrolides | Non-macrolide alternative | ||
|---|---|---|---|---|
| Azithromycin | Clarithromycin | Trimethoprim/sulfamethoxazole | ||
| <1 month | 10 mg/kg daily for 5 days | Not recommended | Not recommended | |
| 1 to <6 months | 10 mg/kg daily for 5 days | 7.5 mg/kg (max 500 mg) twice a day for 7 days | 4+20 mg/kg (max 160+800 mg) twice a day for 7 days | |
| Children ≥6 months | Day 1: 10 mg/kg daily (max 500 mg) Days 2–5: 5 mg/kg daily (max 250 mg) | 7.5 mg/kg (max 500 mg) twice a day for 7 days | 4+20 mg/kg (max 160+800 mg) twice a day for 7 days | |
| Adults | Day 1: 500 mg daily Day 2–5: 250 mg daily | 500 mg twice a day for 7 days | 160+800 mg twice a day for 7 days | |
Pertussis is a vaccine-preventable disease that can be effectively controlled by immunization [24,25]. Completion of the primary vaccination series can prevent > 80% of the cases, and even if infection occurs, the disease is generally milder in vaccinated individuals. Therefore, pertussis vaccination is recommended as a part of routine immunization schedule in all countries [26].
In South Korea, pertussis vaccination is included in the national immunization program and provided free of charge as a part of the standard vaccination schedule for all children. While national schedules vary slightly by country, the standard pertussis vaccination schedule and recommendations for South Korea are presented in Table 2.
Table 2. Pertussis-containing vaccine schedule for children in Korea
| Recommended vaccination age | Minimum interval to next dose | Vaccination | ||
|---|---|---|---|---|
| Primary | 1st | 2 months | At least 6 weeks old | DTaP (or DTaP-containing combination vaccine) |
| 2nd | 4 months | 4 weeks after 1st dose | DTaP (or DTaP-containing combination vaccine) | |
| 3rd | 6 months | 4 weeks after 2nd dose | DTaP (or DTaP-containing combination vaccine) | |
| Booster | 4th | 15–18 months | 6 months for 3rd dose | DTaP |
| 5th | 4–6 years | – | DTaP-IPV (or DTaP) | |
| 6th | 11–12 years | – | Tdap |
Abbreviations: DTaP, diphtheria–tetanus–acellular pertussis; IPV, inactivated poliovirus vaccine
In adults, it is recommended that individuals who have completed the diphtheria–tetanus–acellular pertussis (DTaP) primary and booster series receive at least one dose of Tdap. In pregnant women, Tdap vaccination is strongly recommended during pregnancy, ideally between 27 and 36 weeks of gestation. This approach allows the transfer of protective antibodies to the fetus, offering passive immunity to newborns prior to their first pertussis vaccination at 2 months of age, and is considered one of the most effective strategies for preventing infant pertussis [27–29].
Although the primary DTaP vaccination coverage in South Korea exceeds 90%, booster vaccination rates have been reported to be relatively lower [30]. Notably, maternal Tdap vaccination coverage during pregnancy remains at approximately 60%, underscoring the need for improved education and public awareness campaigns [31].
In the event of confirmed pertussis, patients must be placed under both standard and droplet precautions. Isolation should continue for 5 days after initiating appropriate antibiotic therapy, or 21 days from symptom onset if antibiotics are not administered [4,25].
For close contacts of patients with pertussis, those who are incompletely vaccinated or whose vaccination status is uncertain should receive age-appropriate pertussis-containing vaccines as soon as possible. Regardless of the age or vaccination history, close contacts should receive prophylactic antibiotics to prevent further transmission [4,17,18]. The recommended medications, dosages, and duration of prophylactic use are listed in Table 1 for therapeutic treatment. If prophylactic antibiotics are not administered, the exposed individuals must be monitored closely for pertussis-related symptoms for 21 days following exposure.
Although pertussis is particularly severe in infants younger than 2 months, it can be effectively prevented prior to their first scheduled vaccination through maternal immunization. However, the vaccination rate among pregnant women in South Korea remains suboptimal. In addition, both adult coverage and adolescent booster vaccination rates are considerably lower than primary childhood immunization rates. This gap has contributed to a sharp increase in pertussis cases among adolescents and adults, subsequently leading to transmission to vulnerable populations such as young infants. Therefore, there is an urgent need for enhanced public education and the active promotion of pertussis vaccination among adolescents, adults, and pregnant women. Further research is warranted to evaluate the effectiveness of these interventions and support evidence-based policy development.
It is not a human population study; therefore, approval by the Institutional Review Board or the obtainment of informed consent is not required.
The author has no potential conflict of interest to disclose.
None.
None.
1. Feldmann H, Czub M, Jones S, Dick D, Garbutt M, Grolla A, et al. Emerging and re-emerging infectious diseases. Med Microbiol Immunol 2002;191:63-74.

2. Chen CC and Whitehead A. Emerging and re-emerging infections in children: COVID/ MIS-C, Zika, Ebola, Measles, Varicella, Pertussis … immunizations. Emerg Med Clin North Am 2021;39:453-65.


3. Long SS. Pertussis (Bordetella pertussis and Bordetella parapertussis). In: Kliegman R, Stanton BF, et al. eds. Nelson textbook of pediatrics. 19th ed. Elsevier, 2011:944-8.
4. Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH; Committee on Infectious Diseases, American Academy of Pediatrics. Pertussis (whooping cough). In: Committee on Infectious Diseases, American Academy of Pediatrics, ed. Red book: 2021-2024 report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics, 2021:578-89.
5. Kim KH, Kim YJ, et al. eds. Pertussis (whooping cough). In: Ahn HS, Shin HY, et al. eds. Pediatrics. 12th ed. Mirae N, 2020:425-8.
6. Cherry JD and Heininger U. Chanpter 140 – pertussis and other Bordetella infections. In: Feigin RD, Cherry JD, et al. eds. Feigin and Cherry’s textbook of pediatric infectious diseases. 6th ed. Saunders, 2009:1683-93.
7. Hoppe JE. Neonatal pertussis. Pediatr Infect Dis J 2000;19:244-7.

8. Kilgore PE, Salim AM, Zervos MJ, Schmitt HJ. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev 2016;29:449-86.


9. Domenech de Cellès M and Rohani P. Pertussis vaccines, epidemiology and evolution. Nat Rev Microbiol 2024;22:722-35.

10. Cherry JD. Epidemiology of pertussis. Pediatr Infect Dis J 2006;25:361-2.

11. Di Mattia G, Nicolai A, Frassanito A, Petrarca L, Nenna R, Midulla F. Pertussis: new preventive strategies for an old disease. Paediatr Respir Rev 2019;29:68-73.

12. Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine 2013;31:618-25.

13. Crowcroft NS and Pebody RG. Recent developments in pertussis. Lancet 2006;367:1926-36.

14. Kwantes W, Joynson DH, Williams WO. Bordetella pertussis isolation in general practice: 1977-79 whooping cough epidemic in West Glamorgan. J Hyg (Lond) 1983;90:149-58.


15. Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J 2001;20:380-91.

16. Czumbel I, Quinten C, Lopalco P, Semenza JC; ECDC expert panel working group. Management and control of communicable diseases in schools and other child care settings: systematic review on the incubation period and period of infectiousness. BMC Infect Dis 2018;18:199.


17. Nguyen VTN and Simon L. Pertussis: the whooping cough. Prim Care 2018;45:423-31.

18. Snyder J and Fisher D. Pertussis in childhood. Pediatr Rev 2012;33:412-21.

19. Principi N, Litt D, Terranova L, Picca M, Malvaso C, Vitale C, et al. Pertussis-associated persistent cough in previously vaccinated children. J Med Microbiol 2017;66:1699-702.

20. Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA 1995;273:1044-6.

21. Couzigou C and Flahault A. Is pertussis being considered as a cause of persistent cough among adults? Eur J Epidemiol 2003;18:1013-5.

22. Centers for Disease Control and Prevention. Laboratory testing for pertussis. https://www.cdc. gov/pertussis/php/laboratories/index.html [online] (last visited on 17 November 2024).
23. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2005;1:CD004404.

24. Nieves DJ and Heininger U. Bordetella pertussis. Microbiol Spectr 2016;4:10.1128.

25. Korean Pediatric Society. DTaP, Tdap, Td vaccine. In: Choi EH, ed. Immunization guideline: 2021 report of the Committee on Infectious Diseases. 10th ed. Korean Pediatric Society, 2021:98-118.
26. Kaur G, Danovaro-Holliday MC, Mwinnyaa G, Gacic-Dobo M, Francis L, Grevendonk J, et al. Routine vaccination coverage – worldwide, 2022. MMWR Morb Mortal Wkly Rep 2023;72:1155-61.


27. Leuridan E. Pertussis vaccination in pregnancy: state of the art. Vaccine 2017;35:4453-6.

28. Healy CM. Pertussis vaccination in pregnancy. Hum Vaccin Immunother 2016;12:1972-81.


29. De Weerdt L, Herzog SA, Van Damme P, Maertens K. Timing of pertussis vaccination during pregnancy: evidence and implementation – a systematic review. Vaccine 2024;42:126152.

30. Park B, Lee YK, Cho LY, Go UY, Yang JJ, Ma SH, et al. Estimation of nationwide vaccination coverage and comparison of interview and telephone survey methodology for estimating vaccination status. J Korean Med Sci 2011;26:711-9.


31. Kim C, Pae J, Kim WJ, Jang Y, Wie JH, Park IY, et al. Current status of pertussis vaccination during pregnancy and influencing factors in Korea. Taiwan J Obstet Gynecol 2021;60:273-80.

1. Feldmann H, Czub M, Jones S, Dick D, Garbutt M, Grolla A, et al. Emerging and re-emerging infectious diseases. Med Microbiol Immunol 2002;191:63-74.

2. Chen CC and Whitehead A. Emerging and re-emerging infections in children: COVID/ MIS-C, Zika, Ebola, Measles, Varicella, Pertussis … immunizations. Emerg Med Clin North Am 2021;39:453-65.


3. Long SS. Pertussis (Bordetella pertussis and Bordetella parapertussis). In: Kliegman R, Stanton BF, et al. eds. Nelson textbook of pediatrics. 19th ed. Elsevier, 2011:944-8.
4. Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH; Committee on Infectious Diseases, American Academy of Pediatrics. Pertussis (whooping cough). In: Committee on Infectious Diseases, American Academy of Pediatrics, ed. Red book: 2021-2024 report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics, 2021:578-89.
5. Kim KH, Kim YJ, et al. eds. Pertussis (whooping cough). In: Ahn HS, Shin HY, et al. eds. Pediatrics. 12th ed. Mirae N, 2020:425-8.
6. Cherry JD and Heininger U. Chanpter 140 – pertussis and other Bordetella infections. In: Feigin RD, Cherry JD, et al. eds. Feigin and Cherry’s textbook of pediatric infectious diseases. 6th ed. Saunders, 2009:1683-93.
7. Hoppe JE. Neonatal pertussis. Pediatr Infect Dis J 2000;19:244-7.

8. Kilgore PE, Salim AM, Zervos MJ, Schmitt HJ. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev 2016;29:449-86.


9. Domenech de Cellès M and Rohani P. Pertussis vaccines, epidemiology and evolution. Nat Rev Microbiol 2024;22:722-35.

10. Cherry JD. Epidemiology of pertussis. Pediatr Infect Dis J 2006;25:361-2.

11. Di Mattia G, Nicolai A, Frassanito A, Petrarca L, Nenna R, Midulla F. Pertussis: new preventive strategies for an old disease. Paediatr Respir Rev 2019;29:68-73.

12. Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine 2013;31:618-25.

13. Crowcroft NS and Pebody RG. Recent developments in pertussis. Lancet 2006;367:1926-36.

14. Kwantes W, Joynson DH, Williams WO. Bordetella pertussis isolation in general practice: 1977-79 whooping cough epidemic in West Glamorgan. J Hyg (Lond) 1983;90:149-58.


15. Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J 2001;20:380-91.

16. Czumbel I, Quinten C, Lopalco P, Semenza JC; ECDC expert panel working group. Management and control of communicable diseases in schools and other child care settings: systematic review on the incubation period and period of infectiousness. BMC Infect Dis 2018;18:199.


17. Nguyen VTN and Simon L. Pertussis: the whooping cough. Prim Care 2018;45:423-31.

18. Snyder J and Fisher D. Pertussis in childhood. Pediatr Rev 2012;33:412-21.

19. Principi N, Litt D, Terranova L, Picca M, Malvaso C, Vitale C, et al. Pertussis-associated persistent cough in previously vaccinated children. J Med Microbiol 2017;66:1699-702.

20. Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA 1995;273:1044-6.

21. Couzigou C and Flahault A. Is pertussis being considered as a cause of persistent cough among adults? Eur J Epidemiol 2003;18:1013-5.

22. Centers for Disease Control and Prevention. Laboratory testing for pertussis. https://www.cdc. gov/pertussis/php/laboratories/index.html [online] (last visited on 17 November 2024).
23. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2005;1:CD004404.

24. Nieves DJ and Heininger U. Bordetella pertussis. Microbiol Spectr 2016;4:10.1128.

25. Korean Pediatric Society. DTaP, Tdap, Td vaccine. In: Choi EH, ed. Immunization guideline: 2021 report of the Committee on Infectious Diseases. 10th ed. Korean Pediatric Society, 2021:98-118.
26. Kaur G, Danovaro-Holliday MC, Mwinnyaa G, Gacic-Dobo M, Francis L, Grevendonk J, et al. Routine vaccination coverage – worldwide, 2022. MMWR Morb Mortal Wkly Rep 2023;72:1155-61.


27. Leuridan E. Pertussis vaccination in pregnancy: state of the art. Vaccine 2017;35:4453-6.

28. Healy CM. Pertussis vaccination in pregnancy. Hum Vaccin Immunother 2016;12:1972-81.


29. De Weerdt L, Herzog SA, Van Damme P, Maertens K. Timing of pertussis vaccination during pregnancy: evidence and implementation – a systematic review. Vaccine 2024;42:126152.

30. Park B, Lee YK, Cho LY, Go UY, Yang JJ, Ma SH, et al. Estimation of nationwide vaccination coverage and comparison of interview and telephone survey methodology for estimating vaccination status. J Korean Med Sci 2011;26:711-9.


31. Kim C, Pae J, Kim WJ, Jang Y, Wie JH, Park IY, et al. Current status of pertussis vaccination during pregnancy and influencing factors in Korea. Taiwan J Obstet Gynecol 2021;60:273-80.
