Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
Correspondence to Mi-Na Kim, E-mail: mnkim@amc.seoul.kr
Ann Clin Microbiol 2025;28(2):9. https://doi.org/10.5145/ACM.2025.28.2.3
Received on 8 May 2025, Revised on 22 May 2025, Accepted on 26 May 2025, Published on 16 June 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Diagnostic stewardship aims to improve patient care quality by optimizing diagnostic test utilization, reducing unnecessary testing, and enhancing cost-effectiveness [1,2]. In Korea, diagnostic practices are in the early stages of developing a stewardship program. Although not exclusive to clinical microbiology, diagnostic stewardship programs (DSPs) has gained prominence owing to the global challenge of antimicrobial resistance, aligning with the expansion of antimicrobial stewardship programs (ASPs) [3]. This commentary examines the current status and perspectives of DSPs in clinical microbiology and highlights key strategies and future directions in Korea.
In Korea, ASPs have been actively developing with government support to combat antimicrobial resistance [4]. To implement ASPs, a DSP is required first. For example, the Korea Disease Control and Prevention Agency has been operating a national mandatory surveillance system for six multidrug-resistant organisms (MDROs) as critical priority pathogens of public health since 2010 [5]. This surveillance revealed that a continuous increase in carbapenem-resistant Enterobacterales, especially carbapenemase-producing Enterobacterales (CPE), threatened public health (Fig. 1). Building on these findings, resource allocation and strategic planning, such as ASPs, have been strengthened to address the increasing threats posed by MDROs.
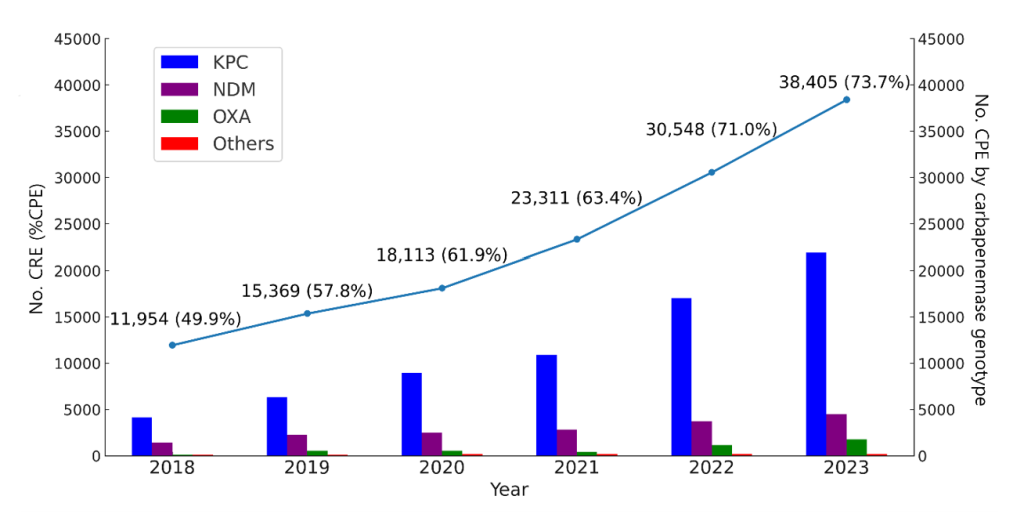
Fig. 1. National surveillance data of carbapenem-resistant Enterobacterales (CRE) in Korea from 2018 to 2023. The line represents the annual number of CRE cases with the percentage of carbapenemaseproducing Enterobacterales (%CPE). The bars indicate the distribution of carbapenemase genotypes including KPC, NDM, OXA, and others among CPE isolates in each year.
In clinical microbiology, diagnostic stewardship efforts have particularly focused on optimizing several key areas: First, the utilization of syndromic testing enabling the simultaneous detection of multiple pathogens from a single sample [6], Second, employment of sample-to-result rapid screening tests to quickly identify pathogens responsible for threatening outbreaks or serious morbidity and mortality, as well as carriers of MDROs to enhance infection control measures. The endemicity and frequent outbreaks of CPEs necessitate the active isolation of carriers via rapid preadmission screening in Korea [7]. Third, there is a strong emphasis on implementing rapid pathogen identification and antimicrobial susceptibility testing (AST) for positive blood cultures [8]. Currently, various molecular diagnostics and rapid phenotypic AST for bloodstream infections are available (Table 1). Therefore, DSPs are becoming increasingly crucial, as it enable clinicians to make informed decisions through timely reporting and alert systems issued by the laboratory (Fig. 2) [9].
Table 1. Rapid pathogen identification and antimicrobial susceptibility tests for application to positive blood cultures or direct blood samples that are currently available or expected to be available in Korea
| Platform/kit (manufacturer) | Species-identification range | Antimicrobial-resistance determination |
|---|---|---|
| Molecular diagnostics | ||
| cobas® ePlex BCID Panels (Roche Diagnostics) | BCID-GP: 20 GP with Pan-GN, Pan-Candida BCID-GN: 21 GN with Pan-GP, Pan-Candida BCID-FP: 15 fungal organisms | mecA, mecC, vanA, vanB, CTX‑M, IMP, KPC, NDM, OXA‑23/48, VIM |
| BioFire® FilmArray BCID2 (bioMérieux) | 33 organism targets: 11 GP, 15 GN, 7 yeasts | mecA/C, MREJ, vanA/B, KPC, NDM, VIM, IMP, OXA‑48‑like, CTX‑M, mcr-1 |
| VERIGENE® BCID Panels (DiaSorin) | BC‑GP: 13 GP genus/species BC‑GN: 9 GN genus/species | BC‑GP: mecA, vanA, vanB BC‑GN: CTX‑M, IMP, KPC, NDM, OXA, VIM |
| T2Candida® Panel (T2 Biosystems)a) | 5 Candida species directly from whole blood | None |
| MALDI Biotyper (Bruker) | Full range cover, ≥ 4,000 bacterial and fungal species – MBT Sepsityper (IVD), ≥425 microorganisms from positive blood cultures | Carbapenemase and cephalosporinase using MBT-STAR (IVD) Colistin resistance using MBT Lipid Xtract (RUO) Detection of blaKPC and cfiA using MBT HT Subtyping (IVD) |
| Vitek MS (bioMérieux) | Full range cover, ≥ 1,000 bacterial and fungal species | None |
| Rapid phenotypic AST | Usually combined with direct species identification from positive blood cultures by MALDI-TOF | |
| dRAST (Quantamatrix Inc.) | GN and GP panel based on gram-stained microscopy of positive blood culture bottles | Phenotypic MICs based on microscopic imaging for 17–19 antimicrobials in 5–7 h |
| Accelerate PhenoTest BC kit (Accelerate Diagnostics, Inc.) | 14 common bloodstream pathogens identified in 2 h | Phenotypic MICs based on microscopic imaging for 12–16 antimicrobials in 7 h |
| Blood culture-GN (PhAST)b) | GN panel based on gram-stained microscopy of positive blood culture bottles | Phenotypic MICs based on microscopic imaging for 10 antimicrobials in 1 h |
| VITEK REVEAL (bioMérieux) | GN panel based on gram-stained microscopy of positive blood culture bottles | Phenotypic MICs based on sensing volatile organic metabolite produced by bacterial growth for 17–19 antimicrobials in 5 h |
| EUCAST RAST | 8 common bloodstream pathogens | Disk diffusion in 4–8 h or 16–20 h |
| CLSI RAST | Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter species | Disk diffusion in 8–10 h or 16–18 h |
a) Under process for IVD approval of Korea Ministry of Food and Drug Safety; b) Under process for IVD approval of US Food and Drug Administration. Abbreviations: MALDI, matrix-assisted laser desorption ionization; GP, gram-positive; GN, gram-negative; IVD, in vitro diagnostics; RUO, research use only; MS, mass spectrometry; AST, antimicrobial susceptibility testing; RAST, rapid antimicrobial susceptibility testing; MALDI-TOF, MALDI time-of-flight; MIC, minimum inhibitory concentration; EUCAST, European Committee in Antimicrobial Susceptibility Testing; CLSI, Clinical and Laboratory Standards Institute.
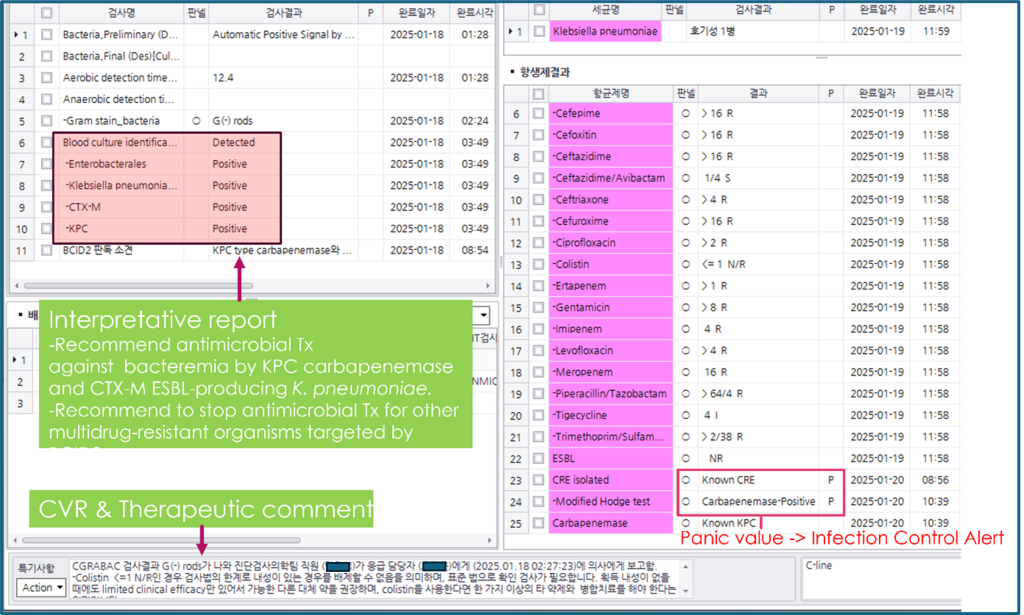
Fig. 2. Application of diagnostic stewardship to blood culture practice in a clinical microbiology laboratory. After BACTEC loading at 1-17 13:07, there were six moments of reports including a critical value report (CVR) and an infection control alert until the final report: (1) Positive signal at 1-18 01:28, (12.4 h from an aerobic bottle); (2) Gram stain results as gram-negative rods seen at 1-18 02:24; (3) Call to clinician at 1-18 02:27 recorded as a CVR, which initiates an automatic consult with infectious disease specialists for antimicrobial stewardship program, 3. KPC/ CTX-M producing Klebsiella pneumoniae positive by BCID2 at 1-18 03:49; (4) Species identification and antimicrobial susceptibility test using colony at 1-19 11:59; (5) Phenotyping and genotyping of carbapenemase at 1-20 10:39; (6) A panic value for issuing an infection control alert.
Although advances in diagnostic technologies have resolved various issues associated with DSP, several challenges remain. The increasing availability of rapid and convenient molecular test kits can lead to their overuse, misuse, and potential underutilization [9]. For example, Clostridioides difficile infection (CDI) control and monitoring are process indicators of the national ASP actions in Korea [5]. Polymerase chain reaction (PCR)-based diagnosis contributes to overdiagnosis and subsequent overtreatment with antibiotics. Therefore, two-step methods, such as PCR followed by toxin enzyme immunoassay (EIA) or glutamate dehydrogenase EIA followed by toxin EIA and/or PCR for discordant results, are preferred for appropriate patient management, minimizing unnecessary antibiotic exposure, and mitigating the risks associated with CDI diagnosis and treatment [10]. Therefore, the significance of DSP throughout the entire testing process, spanning the pre-analytical, analytical, and post-analytical phases, is becoming increasingly apparent.
Looking ahead, collaborative efforts between clinical microbiologists and infectious disease specialists are expected to play a crucial role in improving patient care [9]. In severe infections, such as bacteremia, timely intervention by clinical microbiologists is critical to ensuring appropriate antimicrobial use and improving overall patient outcomes. Thus, diagnostic stewardship in clinical microbiology involves a multifaceted approach, balancing technological advancements with judicious utilization and fostering interdisciplinary collaboration to optimize patient care and combat antimicrobial resistance.
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
No potential conflicts of interest relevant to this article were reported.
None.
None.
1. Clinical and Laboratory Standards Institute. Developing and managing a medical laboratory (test) utilization management program. CLSI GP49. 1st ed. Wayne, PA; CLSI: 2017.
2. Singh HK, Claeys KC, Advani SD, Ballam YJ, Penney J, Schutte KM, et al. Diagnostic stewardship to improve patient outcomes and healthcare-associated infection (HAI) metrics. Infect Control Hosp Epidemiol 2024;45:405-11.


3. Zakhour J, Haddad SF, Kerbage A, Wertheim H, Wertheim H, Voss A, et al. Diagnostic stewardship in infectious diseases: a continuum of antimicrobial stewardship in the fight against antimicrobial resistance. Int J Antimicrob Agents 2023;62:106816.

4. Korean Disease Control and Prevention Agency. 항생제 적정사용 관리 시범사업 및 평가 지침(Antimicrobial Stewardship Pilot Project and Evaluation Guidelines). https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&list_no=726164&act=view [Online] (last visited on 10 October 2024).
5. Lee H, Lee S, Jung YH, Choi J, Park SK. Characteristics of notified carbapenem-resistant Enterobacterales cases in the Republic of Korea, 2023. Public Health Wkly Rep 2025;18:7589.
6. Lewinski MA, Alby K, Babady NE, Butler-Wu SM, Bard JD, Greninger AL, et al. Exploring the utility of multiplex infectious disease panel testing for diagnosis of infection in different body sites: a Joint Report of the Association for Molecular Pathology, American Society for Microbiology, Infectious Diseases Society of America, and Pan American Society for Clinical Virology. J Mol Diagn 2023;25:857-75.


7. Jeong H, Hyun J, Lee Y. Epidemiological characteristics of carbapenemase-producing Enterobacteriaceae outbreaks in the Republic of Korea between 2017 and 2022. Osong Public Health Res Perspect 2023;14:312-20.


8. Fabre V, Carroll KC, Cosgrove SE. Blood culture utilization in the hospital setting: a call for diagnostic stewardship. J Clin Microbiol 2022;60:e0100521.


9. Kim MN. Diagnostic stewardship in clinical microbiology. In: Kim MN, Uh Y, et al. eds. Clinical Microbiology. 1st ed, Paju; Koonja Publishing Inc., 2025:40-5.
10. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55.

1. Clinical and Laboratory Standards Institute. Developing and managing a medical laboratory (test) utilization management program. CLSI GP49. 1st ed. Wayne, PA; CLSI: 2017.
2. Singh HK, Claeys KC, Advani SD, Ballam YJ, Penney J, Schutte KM, et al. Diagnostic stewardship to improve patient outcomes and healthcare-associated infection (HAI) metrics. Infect Control Hosp Epidemiol 2024;45:405-11.


3. Zakhour J, Haddad SF, Kerbage A, Wertheim H, Wertheim H, Voss A, et al. Diagnostic stewardship in infectious diseases: a continuum of antimicrobial stewardship in the fight against antimicrobial resistance. Int J Antimicrob Agents 2023;62:106816.

4. Korean Disease Control and Prevention Agency. 항생제 적정사용 관리 시범사업 및 평가 지침(Antimicrobial Stewardship Pilot Project and Evaluation Guidelines). https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&list_no=726164&act=view [Online] (last visited on 10 October 2024).
5. Lee H, Lee S, Jung YH, Choi J, Park SK. Characteristics of notified carbapenem-resistant Enterobacterales cases in the Republic of Korea, 2023. Public Health Wkly Rep 2025;18:7589.
6. Lewinski MA, Alby K, Babady NE, Butler-Wu SM, Bard JD, Greninger AL, et al. Exploring the utility of multiplex infectious disease panel testing for diagnosis of infection in different body sites: a Joint Report of the Association for Molecular Pathology, American Society for Microbiology, Infectious Diseases Society of America, and Pan American Society for Clinical Virology. J Mol Diagn 2023;25:857-75.


7. Jeong H, Hyun J, Lee Y. Epidemiological characteristics of carbapenemase-producing Enterobacteriaceae outbreaks in the Republic of Korea between 2017 and 2022. Osong Public Health Res Perspect 2023;14:312-20.


8. Fabre V, Carroll KC, Cosgrove SE. Blood culture utilization in the hospital setting: a call for diagnostic stewardship. J Clin Microbiol 2022;60:e0100521.


9. Kim MN. Diagnostic stewardship in clinical microbiology. In: Kim MN, Uh Y, et al. eds. Clinical Microbiology. 1st ed, Paju; Koonja Publishing Inc., 2025:40-5.
10. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55.
