1Department of Laboratory Medicine, 2Research Center, 3Department of Infectious Disease, National Health Insurance Service Ilsan Hospital, Goyang, Korea
Correspondence to Young Ah Kim, E-mail: yakim@nhimc.or.kr
Ann Clin Microbiol 2025 December;28(4):20. https://doi.org/10.5145/ACM.2025.28.4.1
Received on 22 July 2025, Revised on 21 October 2025, Accepted on 23 October 2025, Published on 20 November 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Background: Diagnostic tests are essential for accurate disease identification and monitoring treatment responses. This study aimed to assess the factors influencing the requests for microbial diagnostic tests in patients with various infections.
Methods: Using tailored data from the National Health Insurance Big Data, we examined the usage patterns of microbiological tests among patients with pulmonary tuberculosis (TB) and major bacterial infections between 2020 and 2022, excluding overlapping and complex infections (n = 8,268,992). The types of bacterial infections included pneumonia, sepsis, urinary tract infections, and soft tissue infections, for which bacterial culture and antimicrobial susceptibility tests were performed. For pulmonary TB cases, acid-fast bacillus smears, mycobacterial cultures, and molecular diagnostic tests were performed. Multivariate analysis was used to identify the factors influencing the prescription of microbiological diagnostic tests, considering variables such as age, sex, Charlson comorbidity index, underlying disease, type of medical institution, residential area, insurance quintile, and disability status.
Results: Requests for TB-related and bacterial infection-related tests varied according to multiple factors, including sex, age, insurance quintile, residential area, presence or absence of disability, disease severity, type of medical institution admission, chemotherapy, steroid use, comorbid conditions, and underlying diseases.
Conclusions: This study provides a rare analysis of large-scale national data on various factors affecting test prescriptions, which could provide useful data for improving policies for appropriate test prescriptions.
Big data, Bacterial infections, Communicable diseases, Pulmonary tuberculosis, Routine diagnostic tests
Pulmonary tuberculosis (TB), pneumonia, sepsis, urinary tract infection (UTI), and soft tissue infection (STI) significantly affect public health and can lead to mortality, morbidity, and disability, thereby lowering overall societal health standards.
Pulmonary TB remains a critical public health concern, accounting for over 10 million cases and 1.6 million deaths worldwide by 2021 [1]. TB-related diagnostic practices include acid-fast bacillus (AFB) smears, mycobacterial cultures, and molecular testing [2]. Bacterial culture and antimicrobial susceptibility testing (AST) are fundamental diagnostic procedures for suspected infectious diseases [3].
This study aimed to evaluate the current patterns of microbiological test requests for diagnosing pulmonary TB and major bacterial infections to determine the factors influencing these test prescriptions.
This nationwide, retrospective cohort study utilized data from the Korean National Health Insurance (NHI) Customized Data Service.
This nationwide population-based retrospective study used data from the National Healthcare System of the Republic of Korea. The study period included all patient encounters and diagnostic test requests recorded between January 2020 and December 2022. This setting encompasses all types of medical institutions covered by the national insurance program, including senior general hospitals, general hospitals, clinics, and nursing hospitals.
The study population included all patients within the NHI system who were diagnosed with one of the five infectious diseases: pulmonary TB, pneumonia, sepsis, UTI, or STI. The patients were identified based on their corresponding diagnostic codes from the Korean Standard Classification of Diseases (Supplementary Table 1 in the online-only Data Supplement). The types of bacterial infections were selected according to the Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definitions for specific types of infections [4]. For hospitalized patients, repeated admissions within the same hospitalization period were considered as single episodes.
To eliminate the influence of duplicate cases, only the first occurrence in each patient during the study period was included. Patients with complex infections, defined as those with more than two infectious diseases, were excluded, and only those with a single infection (one diagnosis) were analyzed.
The primary data source for this study was the NHI Customized Data Service. This comprehensive administrative database contains nationwide claims data for a vast majority of the South Korean population.
The analysis focused on a primary outcome variable (the prescription of a microbial diagnostic test). The predictor variables, or factors assessed for their influence on test ordering, included a range of demographic, clinical, and social characteristics.
Diagnostic tests: the study assessed the prescription patterns of AFB smear, mycobacterium culture, and real-time polymerase chain reaction (molecular diagnostic tests) for mycobacteria in patients suspected of having pulmonary TB by referencing the NHI diagnostic codes (Supplementary Table 2 in the online-only Data Supplement). In patients suspected of having pneumonia, sepsis, UTI, or STI, the utilization of bacterial cultures and AST was reviewed (Supplementary Table 2 in the online-only Data Supplement). Monthly trends in test requests were presented and standardized per one million patients.
Patient demographics: age, sex, residential area, and disability status. Age groups were defined as 0-19 years at the time of diagnosis, with subsequent ages classified into 10-year intervals. Residential areas were determined by resident registration and were classified into eight metropolitan cities and nine administrative districts. Additionally, residential locations were analyzed by region, with three categories defined: region 1 (metropolitan areas: Seoul and Gyeonggi), region 2 (metropolitan cities: Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan), and region 3 (Sejong, Gangwon, Chungbuk, Chungnam, Jeollabuk, Jeollanam, Gyeongbuk, Gyeongnam, and Jeju). The presence or absence of disability was determined based on medical insurance disability codes.
Socioeconomic status: insurance quintile, which reflects income level. Income quintiles were categorized into medical benefits and the 1st to 4th quintiles, based on household insurance premiums divided into five groups representing 20 percentile increments in economic status. Insurance eligibility categories included region, workplace, and medical benefits.
Clinical severity and history: the Charlson Comorbidity Index (CCI) was used to measure the overall disease severity and presence of specific underlying diseases. The CCI serves as a measure of disease severity; it assigns specific weights to 19 specified conditions and the sum of these weights produces a total score [5]. CCI scores, calculated from health insurance claims data, were grouped into 1, 2, and 3 or more. The presence of comorbidities, including solid cancer, hematologic malignancy, organ transplantation, human immunodeficiency virus (HIV) infection, diabetes, alcohol dependence, and malnutrition, was assessed for six months preceding the diagnosis of infectious diseases by referencing the NHI diagnostic codes (Supplementary Table 1 in the online-only Data Supplement). Chemotherapy and steroid use were identified based on prescription codes for NHI-authorized therapeutic medications.
Healthcare setting denotes the category of medical institution where the patient was treated. Medical institutions were classified as senior general hospitals, general hospitals (more than 100 beds), hospitals (between 30 and 100 beds), clinics (fewer than 30 beds), nursing hospitals (long-term care facilities for elderly patients with underlying diseases, those requiring guardians, and those with cancer), and others. Senior general hospitals specifically deliver advanced medical care for severe diseases, as designated by the Ministry of Health and Welfare [6]. The ‘others’ category included dental hospitals, health centers, child health centers, and oriental medicine clinics. The initial medical institution was designated in case of patient transfer.
Condition-specific factors: Pregnancy status was considered for UTIs [7], while diagnoses such as necrotizing fasciitis, pyogenic arthritis, Clostridium muscle necrosis, and animal bites were assessed for STIs [8]. The presence or absence of diagnostic codes for pregnancy, necrotizing fasciitis, pyogenic arthritis, Clostridium muscle necrosis, and open wounds caused by bites was assessed within one month (Supplementary Table 1 in the online-only Data Supplement).
To minimize selection bias and misclassification, patients with more than two infectious diseases were excluded, and only the first infection episode for each patient during the study period was analyzed. Confounding factors were addressed using multivariate logistic regression analysis.
This study included all eligible patients with specified infectious diseases recorded in the NHI database between January 2020 and December 2022 to maximize the statistical power and generalizability.
Multivariate analysis was performed to control for confounding variables and to identify the determinants influencing the prescription of microbial diagnostic tests. Logistic regression was used for statistical analysis, and significance was set at P < 0.05. All analyses were performed using the SAS software (version 9.4; SAS Institute). The NHI claims database contains minimal missing data on the core demographic and diagnostic variables. The analysis was performed on a complete-case basis, excluding a small number of records with missing data for any variables included in the model.
During the study period, 8,803,283 patients were identified with a diagnosis code for infectious diseases. Of these, 222,703 were excluded because of complex or overlapping infections, and 311,588 were excluded because this was not their first recorded episode, resulting in a final analytical cohort of 8,268,992 patients.
The monthly number of TB-related tests, such as AFB smears, mycobacterium cultures, and molecular diagnostic tests (the number of tests displayed per million patients), showed a continuous increase, with a slight decline in April 2022 (Fig. 1).

Fig. 1. Trends in requests for tuberculosis-related tests. N, number; AFB, acid-fast bacilli; TB, tuberculosis.
Prescriptions were most frequent among patients with suspected pneumonia, with a peak monthly number of bacterial cultures and ASTs (per one million patients) in December 2021. However, the number of bacterial cultures and ASTs (per one million patients) consistently declined after June 2022, with the lowest levels in December 2022 (Fig. 2A). The number of bacterial cultures and ASTs (per one million patients) peaked in 2022 and plateaued in patients with suspected sepsis (Fig. 2B). Among patients with suspected UTI, the numbers of bacterial cultures and ASTs (per one million patients) dropped sharply in 2022 and reached a plateau thereafter (Fig. 2C). The number of bacterial cultures and ASTs (per one million patients) showed a continuous decrease after 2022 in patients with suspected STI (Fig. 2D).
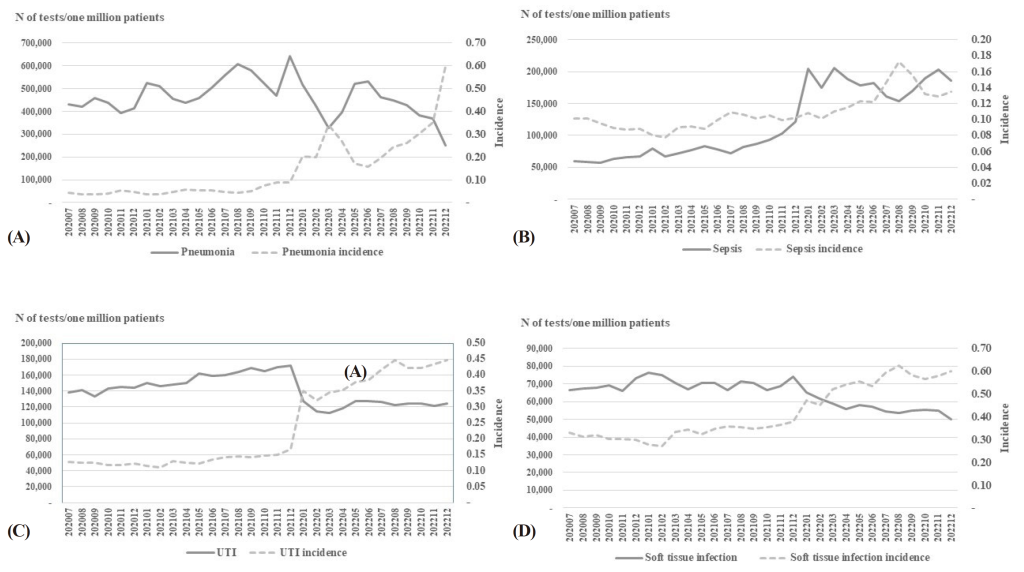
Fig. 2. Trends in requests for bacterial cultures and antimicrobial susceptibility tests for patients suspected of (A) pneumonia, (B) sepsis, (C) UTI, (D) soft tissue infection. N, number; UTI, urinary tract infection.
TB-related tests (AFB smear, mycobacterium culture, and molecular diagnostic tests) were more frequently requested among males, older adults (30-39 years and over 60 years), those with medical benefits, residents outside metropolitan areas, individuals without disabilities, patients with a CCI score greater than 2, nursing hospital admissions, patients receiving chemotherapy for solid cancer, those with hematologic malignancy, organ transplant recipients, individuals with alcohol dependency, and those with malnutrition (Table 1). However, HIV infection and diabetes were associated with fewer requests for TB-related testing.
Table 1. Factors affecting requests for tuberculosis-related tests: multivariate analysis
| Characteristics | Variables | Total (n) | Incidence rate (%) | AFB smear | Mycobacterium culture | Molecular diagnostic test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | ||||
| Sex | Male | 260,824 | 1.01 | 1 | – | – | 1 | – | – | 1 | – | – |
| Female | 421,374 | 1.64 | 0.535 | 0.518 – 0.553 | <0.0001 | 0.533 | 0.516 – 0.550 | <0.0001 | 0.522 | 0.505 – 0.540 | <0.0001 | |
| Age (yr) | ≤ 19 | 42,344 | 0.52 | 1 | – | – | 1 | – | – | 1 | – | – |
| 20-29 | 41,066 | 0.62 | 2.733 | 2.322 – 3.217 | 0.1562 | 2.637 | 2.245 – 3.098 | 0.2753 | 2.746 | 2.317 – 3.254 | 0.2527 | |
| 30-39 | 53,493 | 0.77 | 2.526 | 2.157 – 2.957 | <0.0001 | 2.448 | 2.095 – 2.860 | 0.0005 | 2.715 | 2.305 – 3.197 | 0.0930 | |
| 40-49 | 69,234 | 0.85 | 2.772 | 2.379 – 3.230 | 0.1429 | 2.647 | 2.276 – 3.078 | 0.1679 | 2.862 | 2.441 – 3.356 | 0.8762 | |
| 50-59 | 110,809 | 1.29 | 3.007 | 2.591 – 3.491 | 0.0692 | 2.844 | 2.455 – 3.294 | 0.1385 | 3.008 | 2.576 – 3.514 | 0.0527 | |
| 60-69 | 152,864 | 2.11 | 3.092 | 2.667 – 3.585 | 0.0007 | 2.899 | 2.505 – 3.354 | 0.0099 | 2.925 | 2.507 – 3.414 | 0.4200 | |
| 70-79 | 134,527 | 3.73 | 3.698 | 3.187 – 4.291 | <0.0001 | 3.411 | 2.946 – 3.949 | <0.0001 | 3.484 | 2.984 – 4.069 | <0.0001 | |
| ≥ 80 | 77,861 | 3.79 | 7.338 | 6.324 – 8.515 | <0.0001 | 6.819 | 5.888 – 7.897 | <0.0001 | 7.139 | 6.113 – 8.336 | <0.0001 | |
| Insurance quintile | Medical benefits | 51,784 | 3.40 | 1 | – | – | 1 | – | – | 1 | – | – |
| 1st | 140,810 | 1.39 | 0.958 | 0.901 – 1.018 | 0.0468 | 0.978 | 0.918 – 1.040 | 0.0268 | 0.982 | 0.920 – 1.048 | 0.0201 | |
| 2nd | 120,629 | 1.15 | 0.989 | 0.928 – 1.053 | 0.0002 | 1.009 | 0.946 – 1.075 | <0.0001 | 0.995 | 0.931 – 1.064 | 0.0034 | |
| 3rd | 148,033 | 1.17 | 0.925 | 0.870 – 0.983 | 0.8406 | 0.943 | 0.886 – 1.004 | 0.9962 | 0.960 | 0.900 – 1.024 | 0.3086 | |
| 4th | 220,942 | 1.31 | 0.784 | 0.740 – 0.831 | <0.0001 | 0.802 | 0.755 – 0.851 | <0.0001 | 0.799 | 0.751 – 0.850 | <0.0001 | |
| Residential area | Region 3 | 240,611 | 1.49 | 1 | – | – | 1 | – | – | 1 | – | – |
| Region 2 | 156,328 | 1.21 | 0.848 | 0.814 – 0.884 | 0.0055 | 0.842 | 0.808 – 0.879 | 0.0001 | 0.888 | 0.851 – 0.928 | 0.4749 | |
| Region 1 | 285,259 | 1.20 | 0.800 | 0.772 – 0.829 | <0.0001 | 0.824 | 0.795 – 0.854 | <0.0001 | 0.812 | 0.782 – 0.843 | <0.0001 | |
| Disability | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 0.899 | 0.862 – 0.937 | <0.0001 | 0.904 | 0.866 – 0.944 | <0.0001 | 0.904 | 0.865 – 0.945 | <0.0001 | |||
| Disease severity (CCI) | 0 | 1 | – | – | 1 | – | – | 1 | – | – | ||
| 1 | 1.205 | 1.137 – 1.278 | 0.9317 | 1.188 | 1.120 – 1.260 | 0.7244 | 1.164 | 1.095 – 1.238 | 0.1777 | |||
| 2 | 1.289 | 1.212 – 1.372 | <0.0001 | 1.278 | 1.200 – 1.360 | 0.0001 | 1.278 | 1.198 – 1.364 | 0.0001 | |||
| ≥ 3 | 1.350 | 1.274 – 1.432 | <0.0001 | 1.344 | 1.267 – 1.425 | <0.0001 | 1.358 | 1.278 – 1.443 | <0.0001 | |||
| Types of medical institutions | Senior general hospitals | 1 | – | – | 1 | – | – | 1 | – | – | ||
| General hospitals | 0.779 | 0.753 – 0.806 | <0.0001 | 0.778 | 0.752 – 0.806 | <0.0001 | 0.848 | 0.819 – 0.879 | <0.0001 | |||
| Hospitals | 0.385 | 0.362 – 0.411 | <0.0001 | 0.352 | 0.329 – 0.376 | <0.0001 | 0.269 | 0.249 – 0.290 | 0.0893 | |||
| Clinics | 0.014 | 0.012 – 0.015 | <0.0001 | 0.013 | 0.012 – 0.014 | <0.0001 | 0.013 | 0.012 – 0.014 | <0.0001 | |||
| Nursing hospital | 1.543 | 1.396 – 1.705 | <0.0001 | 1.296 | 1.167 – 1.440 | <0.0001 | 1.153 | 1.030 – 1.291 | <0.0001 | |||
| Othersa) | 0.084 | 0.054 – 0.129 | <0.0001 | 0.069 | 0.043 – 0.112 | <0.0001 | 0.063 | 0.037 – 0.107 | <0.0001 | |||
| Chemotherapy in solid cancer | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 1.075 | 1.016 – 1.136 | 0.0114 | 1.080 | 1.021 – 1.143 | 0.0069 | 1.027 | 0.967 – 1.089 | 0.3854 | |||
| Hematologic malignancy | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 1.125 | 1.016 – 1.244 | 0.0233 | 1.149 | 1.038 – 1.271 | 0.0073 | 1.141 | 1.026 – 1.268 | 0.015 | |||
| Organ transplantation | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 1.269 | 1.082 – 1.490 | 0.0035 | 1.324 | 1.131 – 1.551 | 0.0005 | 1.149 | 0.967 – 1.367 | 0.1151 | |||
| HIV infection | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 0.756 | 0.606 – 0.943 | 0.0133 | 0.791 | 0.637 – 0.984 | 0.0349 | 0.717 | 0.569 – 0.903 | 0.0047 | |||
| Diabetes | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 0.351 | 0.338 – 0.364 | <0.0001 | 0.350 | 0.337 – 0.363 | <0.0001 | 0.338 | 0.326 – 0.352 | <0.0001 | |||
| Alcohol dependence | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 1.735 | 1.542 – 1.952 | <0.0001 | 1.738 | 1.543 – 1.957 | <0.0001 | 1.671 | 1.475 – 1.892 | <0.0001 | |||
| Malnutrition | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 1.883 | 1.764 – 2.010 | <0.0001 | 1.845 | 1.726 – 1.972 | <0.0001 | 1.707 | 1.590 – 1.832 | <0.0001 | |||
| Steroid treatment | No | 1 | – | – | 1 | – | – | 1 | – | – | ||
| Yes | 1.001 | 0.969 – 1.033 | 0.9636 | 0.996 | 0.964 – 1.029 | 0.8034 | 1.014 | 0.980 – 1.048 | 0.4228 | |||
Bold form: statistically significant.
a)Others included dental hospitals, healthcare centers, child health centers, and oriental medicine clinics.
Abbreviations: AFB, acid-fast bacilli; OR, odds ratio; CI, confidence interval; CCI, Charlson Comorbidity Index; HIV, human immunodeficiency virus.
Male sex, advanced age (> 80 years), receiving medical benefits, living in metropolitan areas, having a disability, a CCI score greater than 1, admission to senior general hospitals, receiving chemotherapy for solid cancer, hematologic malignancy, organ transplantation, alcohol dependency, malnutrition, and steroid use were all significant factors. Conversely, patients with diabetes or an HIV infection were less likely to undergo these tests (Table 2).
Table 2. Factors affecting bacterial culture and antimicrobial susceptibility tests: multivariate analysis
| Characteristics | Variables | Pneumonia | Sepsis | Urinary tract infection | Soft tissue infection | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Incidence rate (%) | OR | 95% CI | P-value | Total (n) | Incidence rate (%) | OR | 95% CI | P-value | Total (n) | Incidence rate (%) | OR | 95% CI | P-value | Total (n) | Incidence rate (%) | OR | 95% CI | P-value | ||
| Sex | Male | 1,158,288 | 4.50 | 1 | – | – | 787,410 | 3.06 | 1 | – | – | 1,181,580 | 4.59 | 1 | – | – | 2,965,689 | 11.52 | 1 | – | – |
| Female | 1,331,530 | 5.19 | 0.789 | 0.782 – 0.796 | <0.0001 | 1,177,562 | 4.59 | 0.856 | 0.840 – 0.872 | <0.0001 | 2,827,982 | 11.02 | 1.440 | 1.426 – 1.455 | <0.0001 | 4,439,665 | 17.29 | 0.781 | 0.773-0.789 | <.0001 | |
| Age (yr) | ≤ 19 | 878,496 | 10.77 | 1 | – | – | 369,517 | 4.53 | 1 | – | – | 363,021 | 4.45 | 1 | – | – | 1,034,285 | 12.68 | 1 | – | – |
| 20-29 | 154,422 | 2.33 | 0.245 | 0.237 – 0.254 | <0.0001 | 218,816 | 3.30 | 0.148 | 0.139 – 0.158 | <0.0001 | 345,349 | 5.21 | 0.269 | 0.263 – 0.276 | <0.0001 | 813,367 | 12.28 | 0.775 | 0.747-0.804 | <0.0001 | |
| 30-39 | 196,214 | 2.83 | 0.275 | 0.267 – 0.283 | <0.0001 | 225,691 | 3.26 | 0.154 | 0.146 – 0.163 | <0.0001 | 433,155 | 6.26 | 0.218 | 0.213 – 0.224 | <0.0001 | 885,662 | 12.79 | 0.729 | 0.705-0.754 | <0.0001 | |
| 40-49 | 216,575 | 2.65 | 0.306 | 0.299 – 0.314 | <0.0001 | 233,956 | 2.86 | 0.193 | 0.184 – 0.203 | <0.0001 | 522,278 | 6.39 | 0.237 | 0.232 – 0.242 | <0.0001 | 972,174 | 11.89 | 0.732 | 0.71-0.755 | <0.0001 | |
| 50-59 | 232,689 | 2.70 | 0.351 | 0.344 – 0.358 | <0.0001 | 279,990 | 3.25 | 0.220 | 0.211 – 0.229 | <0.0001 | 698,113 | 8.10 | 0.265 | 0.260 – 0.270 | <0.0001 | 1,175,666 | 13.64 | 0.785 | 0.762-0.807 | <0.0001 | |
| 60-69 | 307,508 | 4.24 | 0.415 | 0.407 – 0.422 | <0.0001 | 298,548 | 4.12 | 0.283 | 0.273 – 0.294 | <0.0001 | 762,451 | 10.52 | 0.296 | 0.291 – 0.302 | <0.0001 | 1,219,787 | 16.83 | 0.949 | 0.923-0.976 | 0.6523 | |
| 70-79 | 256,790 | 7.11 | 0.615 | 0.604 – 0.626 | <0.0001 | 202,898 | 5.62 | 0.408 | 0.393 – 0.424 | <0.0001 | 529,278 | 14.66 | 0.410 | 0.402 – 0.418 | <0.0001 | 808,478 | 22.40 | 1.310 | 1.273-1.347 | <0.0001 | |
| ≥ 80 | 247,124 | 12.03 | 1.218 | 1.197 – 1.239 | <0.0001 | 135,556 | 6.60 | 0.586 | 0.565 – 0.609 | <0.0001 | 355,917 | 17.33 | 0.821 | 0.806 – 0.836 | <0.0001 | 495,935 | 24.15 | 1.672 | 1.625-1.721 | <0.0001 | |
| Insurance quintile | Medical benefits | 117,885 | 7.74 | 1 | – | – | 95,896 | 6.30 | 1 | – | – | 189,335 | 12.44 | 1 | – | – | 317,294 | 20.84 | 1 | – | – |
| 1st | 500,468 | 4.95 | 0.824 | 0.808 – 0.839 | 0.0011 | 407,336 | 4.03 | 0.924 | 0.892 – 0.958 | 0.4517 | 874,066 | 8.65 | 0.769 | 0.754 – 0.784 | <0.0001 | 1,566,003 | 15.50 | 0.765 | 0.750-0.780 | <0.0001 | |
| 2nd | 404,582 | 3.87 | 0.845 | 0.829 – 0.861 | 0.0337 | 362,397 | 3.47 | 0.937 | 0.903 – 0.973 | 0.0406 | 742,253 | 7.11 | 0.782 | 0.767 – 0.798 | 0.4451 | 1,398,004 | 13.38 | 0.800 | 0.784-0.816 | 0.6651 | |
| 3rd | 624,273 | 4.94 | 0.826 | 0.811 – 0.841 | 0.0039 | 460,570 | 3.65 | 0.923 | 0.890 – 0.956 | 0.5587 | 915,774 | 7.25 | 0.760 | 0.745 – 0.775 | <0.0001 | 1,737,600 | 13.76 | 0.765 | 0.750-0.780 | <0.0001 | |
| 4th | 842,610 | 5.01 | 0.71 | 0.698 – 0.723 | <0.0001 | 638,773 | 3.80 | 0.814 | 0.787 – 0.842 | <0.0001 | 1,288,134 | 7.66 | 0.653 | 0.641 – 0.665 | <0.0001 | 2,386,453 | 14.20 | 0.692 | 0.679-0.704 | <0.0001 | |
| Residential area | Region 3 | 880,301 | 5.44 | 1 | – | – | 621,568 | 3.84 | 1 | – | – | 1,300,319 | 8.04 | 1 | – | – | 2,367,804 | 14.64 | 1 | – | – |
| Region 2 | 606,751 | 4.71 | 0.958 | 0.947 – 0.968 | <0.0001 | 469,702 | 3.64 | 0.885 | 0.863 – 0.906 | <0.0001 | 993,619 | 7.71 | 0.925 | 0.914 – 0.936 | 0.0485 | 1,770,905 | 13.73 | 0.989 | 0.976-1.003 | <0.0001 | |
| Region 1 | 1,002,766 | 4.20 | 1.020 | 1.010 – 1.030 | <0.0001 | 873,702 | 3.66 | 0.963 | 0.943 – 0.983 | 0.0166 | 1,715,624 | 7.19 | 0.837 | 0.828 – 0.846 | <0.0001 | 3,266,645 | 13.69 | 0.909 | 0.898-0.919 | <0.0001 | |
| Disability | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 1.509 | 1.490 – 1.528 | <0.0001 | 1.387 | 1.354 – 1.421 | <0.0001 | 1.470 | 1.450 – 1.490 | <0.0001 | 1.685 | 1.663-1.706 | <0.0001 | |||||||||
| Disease severity (CCI) | 0 | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| 1 | 1.461 | 1.442 – 1.48 | <0.0001 | 0.992 | 0.963 – 1.023 | <0.0001 | 1.036 | 1.022 – 1.050 | <0.0001 | 1.402 | 1.372-1.433 | <0.0001 | |||||||||
| 2 | 1.546 | 1.519 – 1.574 | <0.0001 | 1.320 | 1.272 – 1.371 | <0.0001 | 1.154 | 1.135 – 1.174 | <0.0001 | 1.759 | 1.718-1.801 | <0.0001 | |||||||||
| ≥ 3 | 1.855 | 1.823 – 1.887 | <0.0001 | 1.772 | 1.712 – 1.834 | <0.0001 | 1.340 | 1.319 – 1.362 | <0.0001 | 3.340 | 3.267-3.415 | <0.0001 | |||||||||
| Type of medical institutions | Senior general hospitals | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| General hospitals | 0.835 | 0.824 – 0.846 | <0.0001 | 0.377 | 0.368 – 0.386 | <0.0001 | 0.912 | 0.900 – 0.924 | <0.0001 | 0.628 | 0.618-0.638 | <0.0001 | |||||||||
| Hospitals | 0.462 | 0.455 – 0.469 | <0.0001 | 0.077 | 0.074 – 0.080 | <0.0001 | 0.481 | 0.473 – 0.489 | <0.0001 | 0.513 | 0.502-0.523 | <0.0001 | |||||||||
| Clinics | 0.065 | 0.064 – 0.066 | <0.0001 | 0.010 | 0.010 – 0.011 | <0.0001 | 0.080 | 0.079 – 0.082 | <0.0001 | 0.197 | 0.195-0.200 | <0.0001 | |||||||||
| Nursing hospital | 0.700 | 0.685 – 0.715 | <0.0001 | 0.784 | 0.761 – 0.808 | <0.0001 | 0.727 | 0.704 – 0.751 | <0.0001 | 1.243 | 1.210-1.277 | <0.0001 | |||||||||
| Othersa) | 0.137 | 0.117 – 0.161 | <0.0001 | 0.023 | 0.017 – 0.031 | <0.0001 | 0.136 | 0.122 – 0.152 | <0.0001 | 0.351 | 0.324-0.380 | <0.0001 | |||||||||
| Chemotherapy in solid cancer | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 1.358 | 1.328 – 1.389 | <0.0001 | 1.751 | 1.686 – 1.819 | <0.0001 | 0.955 | 0.931 – 0.979 | 0.0003 | 1.898 | 1.858-1.940 | <0.0001 | |||||||||
| Hematologic malignancy | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 1.782 | 1.711 – 1.856 | <0.0001 | 1.839 | 1.730 – 1.954 | <0.0001 | 1.182 | 1.118 – 1.250 | <0.0001 | 1.887 | 1.809-1.968 | <0.0001 | |||||||||
| Organ transplantation | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 1.585 | 1.504 – 1.67 | <0.0001 | 1.396 | 1.283 – 1.518 | <0.0001 | 1.664 | 1.575 – 1.758 | <0.0001 | 2.029 | 1.940-2.122 | <0.0001 | |||||||||
| HIV infection | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 0.855 | 0.747 – 0.980 | 0.0243 | 0.536 | 0.418 – 0.688 | <0.0001 | 0.515 | 0.432 – 0.615 | <0.0001 | 0.833 | 0.723-0.960 | 0.0117 | |||||||||
| Diabetes | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 0.899 | 0.889 – 0.909 | <0.0001 | 0.909 | 0.889 – 0.930 | <0.0001 | 0.942 | 0.932 – 0.953 | <0.0001 | 2.613 | 2.573-2.654 | <0.0001 | |||||||||
| Alcohol dependence | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 2.088 | 2.001 – 2.179 | <0.0001 | 1.692 | 1.562 – 1.832 | <0.0001 | 1.845 | 1.759 – 1.935 | <0.0001 | 1.712 | 1.642-1.785 | <0.0001 | |||||||||
| Malnutrition | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 1.927 | 1.883 – 1.971 | <0.0001 | 1.828 | 1.754 – 1.906 | <0.0001 | 1.698 | 1.651 – 1.746 | <0.0001 | 1.765 | 1.717-1.815 | <0.0001 | |||||||||
| Steroid treatment | No | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | ||||||||
| Yes | 1.227 | 1.216 – 1.237 | <0.0001 | 0.911 | 0.894 – 0.928 | <0.0001 | 1.093 | 1.083 – 1.104 | <0.0001 | 1.141 | 1.130-1.153 | <0.0001 | |||||||||
| Pregnancy | No | 1 | – | – | |||||||||||||||||
| Yes | 0.712 | 0.685 – 0.740 | <0.0001 | ||||||||||||||||||
| Necrotizing fasciitis | No | 1 | – | – | |||||||||||||||||
| Yes | 13.21 | 11.912-14.649 | <0.0001 | ||||||||||||||||||
| Pyogenic arthritis | No | 1 | – | – | |||||||||||||||||
| Yes | 3.21 | 3.097-3.328 | <0.0001 | ||||||||||||||||||
| Clostridium muscle necrosis | No | 1 | – | – | |||||||||||||||||
| Yes | 9.751 | 6.077-15.647 | <0.0001 | ||||||||||||||||||
| Open wounds by bites | No | 1 | – | – | |||||||||||||||||
| Yes | 1.199 | 1.182-1.216 | <0.0001 | ||||||||||||||||||
Bold form: statistically significant.
a)Others included dental hospitals, healthcare centers, child health centers, and oriental medicine clinics.
Abbreviations: OR, odds ratio; CI, confidence interval; CCI, Charlson comorbidity index; HIV, human immunodeficiency virus.
Significant factors included male sex, younger age (< 19 years), receiving medical benefits, living outside metropolitan areas, presence of disability, CCI score > 2, senior general hospital admission, chemotherapy for solid cancer, hematologic malignancy, organ transplantation, alcohol dependency, and malnutrition. However, there were fewer requests from patients with HIV infection, diabetes, or steroid use (Table 2).
Significant factors included female sex, younger age (< 19 years), receipt of medical benefits, residence outside metropolitan cities or areas, disability, CCI greater than 1, admission to senior general hospitals, hematologic malignancy, organ transplantation, alcohol dependency, malnutrition, and steroid use. Conversely, lower request rates were observed among the groups undergoing chemotherapy for solid cancers and among those with HIV infection, diabetes, and pregnancy (Table 2).
Significant factors included male sex, older age (> 70 years), receipt of medical benefits, residence outside metropolitan cities or areas, disability, CCI greater than 1, admission to nursing hospitals, chemotherapy for solid cancer, hematological malignancy, organ transplantation, diabetes, alcohol dependency, malnutrition, steroid use, necrotizing fasciitis, pyogenic arthritis, Clostridium muscle necrosis, and open wounds. By contrast, requests were lower among individuals infected with HIV (Table 2).
Requests for TB-related tests consistently increased, whereas those for bacterial tests varied by infection type and peaked for pneumonia. Test ordering was higher in patients with risk factors such as male sex, older age, high disease severity (CCI > 2), and comorbidities such as cancer and malnutrition. Conversely, HIV infection and diabetes were generally associated with fewer requests across most types of infection.
Active microbiological diagnostic tests are required for patients at a higher risk of infection. The known risk factors for pulmonary TB include undernourishment, smoking, alcohol use, diabetes, and HIV [9]. Various risk factors have been identified for bacterial infections. Age, smoking, environmental exposure, malnutrition, previous community-acquired pneumonia (CAP), chronic bronchitis/chronic obstructive pulmonary disease, asthma, functional impairment, poor dental health, immunosuppressive therapy, oral steroids, and treatment with gastric acid-suppressive drugs were definitive risk factors for CAP [10]. Several well-known risk factors for sepsis include multiple comorbidities, polypharmacy, immunosuppression, and indwelling devices [11]. Women are more prone to UTIs because of behavioral factors, susceptibility factors, genetic factors, age-specific factors, pregnancy-related factors, and urinary catheterization [12]. However, there have been no studies on the risk factors for infections, including socioeconomic aspects such as types of medical institutions, residential area, insurance quintile, and disability, in addition to clinical factors. This study aimed to assess the factors influencing requests for microbial diagnostic tests in patients with various infections, which could serve as baseline data for the management of adequate microbiological tests in Korea.
TB-related tests were more frequent in males in this study, although the incidence of female patients was higher. The prevalence of TB is significantly higher among men, and there is strong evidence that men are disadvantaged in seeking and/or accessing TB care in many settings [13]. This finding is inconsistent with that of a previous study and requires further investigation into the affecting factors. In this study, more bacterial infection-related tests were performed on females. Sex differences in bacterial infections have been reported due to differences in immunity, health-seeking behaviors, quality of health care, and adherence to treatment recommendations [14].
In this study, TB-related tests were more frequently requested among patients over 60 years of age, and the incidence rates increased, which is consistent with the high prevalence of pulmonary TB in older patients in Korea [15]. However, it was unexpected that requests for TB-related tests were high in the 30-39 age group, which requires further investigation. Bacterial infection tests were more frequently requested among patients aged > 80 years, and the incidence rate increased rapidly in this group. Elderly individuals are vulnerable to infections because of their increased stay in hospitals (or long-term care facilities) and exposure to drugresistant pathogens [16]. An aging immune system is also associated with bacterial infections [16].
According to the insurance quintile, the group with medical benefits received more TB-related test orders, which is consistent with the finding of a higher TB incidence in this group with medical benefits [15]. In a cross-country regression study, income inequality may create conditions under which TB spreads more easily [17]. The group with medical benefits received more bacterial infection-related test orders and had the highest incidence rate. Poverty increases the risk of contracting infectious diseases, but evidence regarding this relationship is lacking compared to that for TB cases [18].
Disabled patients underwent fewer TB-related tests, emphasizing the need to enhance accessibility to people with disabilities. Patients with a CCI score greater than 2 requested more TB-related tests and were more likely to have nursing hospital admissions, which is likely reflective of an environment where group living increases susceptibility to infections. Bacterial infection-related tests were more frequently performed in general hospitals, possibly because of greater disease severity.
This study demonstrated that clinicians are more likely to prescribe microbiological diagnostic tests to patients with various underlying diseases, such as solid cancer, hematologic malignancy, organ transplantation, alcohol dependence, and malnutrition. Diabetic patients suspected of having STI were requested to undergo bacterial infection-related tests, but those with other types of infection were not. Compared with control subjects without diabetes, patients with diabetes had higher rates of all infections, with the highest incidence rate observed for bone and joint infections, sepsis, and cellulitis [19]. Therefore, a more active workup for sepsis is needed in patients with diabetes. Patients with HIV infection showed fewer requests for diagnostic tests, but this should be interpreted with caution, as the sample size was smaller than that for other comorbidities.
Data inaccuracy may arise because such datasets reflect physician billing activities rather than the actual clinical status of patients. Individuals with different types of medical benefits and variations across residential regions remain unclear and require further investigation. Nevertheless, this study analyzed rare large-scale national data on various factors affecting test prescriptions, which could help select a target group that needs to focus on policies for appropriate test prescriptions.
More studies are needed to explain why lower request rates were observed in pregnant women, because pregnancy is a well-known risk factor for UTI [12]. According to Korean guidelines for UTI management [7], urine culture is not mandatory; however, it is recommended for pregnant women. Considering the increased rate of antibiotic resistance among UTI pathogens in Korea [20], more active urine culture testing is needed, especially in pregnant patients.
Patients diagnosed with necrotizing fasciitis, pyogenic arthritis, Clostridium muscle necrosis, and open wounds were requested to undergo additional microbiological tests, as recommended in the Korean guidelines for STI management [8]. In this study, more tests were requested in patients receiving chemotherapy for solid cancer, hematologic malignancy, organ transplantation, diabetes, alcohol dependency, malnutrition, and steroid use, which is consistent with the guidelines recommending tests for immunocompromised individuals [21].
This study utilized a comprehensive nationwide database: therefore, the findings are highly generalizable to the entire insured population of South Korea. However, caution should be exercised when extrapolating these results to countries with significantly different healthcare, insurance, and reimbursement systems.
This study analyzed rare large-scale national data on various factors affecting test prescriptions, which could provide useful data for improving policies for appropriate test prescriptions.
The following supplementary materials are available on the journal’s website:
This study was approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital (approval number: NHIMC IRB 2024-10-023).
No potential conflicts of interest relevant to this article were reported.
This work was supported by the National Health Insurance Service Ilsan Hospital grant (NHIMC-2024PR-004).
This study used NHIS-NSC data (NHIS-2025-05-08-1-039) from the National Health Insurance Service (NHIS).
1. World Health Organization. Global tuberculosis report 2021. https://www.who.int/publications/i/item/9789240037021 [Online] (last visited on 28 September 2025).
2. Rodrigues C and Vadwai V. Tuberculosis: laboratory diagnosis. Clin Lab Med 2012;32:111-27.

3. Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 2018;67:e1-94.


4. National Healthcare Safety Network. CDC/NHSN Surveillance Definitions for Specific Types of Infections. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf [Online] (last visited on 28 September 2025).
5. Kim KH. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients. J Prev Med Public Health 2010;43:42-9.

6. Ministry of Health and Welfare. Senior general hospitals. https://www.mohw.go.kr/menu.es?mid=a10702030300 [Online] (last visited on 28 September 2025).
7. Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother 2018;50:67-100.


8. Kwak YG, Choi SH, Kim T, Park SY, Seo SH, Kim MB, et al. Clinical guidelines for the antibiotic treatment for community-acquired skin and soft tissue infection. Infect Chemother 2017;49:301-25.


9. Trajman A, Campbell JR, Kunor T, Ruslami R, Amanullah F, Behr MA, et al. Tuberculosis. Lancet 2025;405:850-66.

10. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration 2017;94:299-311.

11. Del Bono V and Giacobbe DR. Bloodstream infections in internal medicine. Virulence 2016;7:353-65.


12. Kaur R and Kaur R. Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad Med J 2021;97:803-12.

13. Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 2016;13:e1002119.


14. Dias SP, Brouwer MC, van de Beek D. Sex and gender differences in bacterial infections. Infect Immun 2022;90:e0028322.


15. Lee H, Kim J, Kim J, Park YJ, Jeong H, Kim H, et al. Tuberculosis notification status in the Republic of Korea, 2024. Public Health Wkly Rep 2025;18(Suppl 11):S6-S22.
16. Kline KA and Bowdish DM. Infection in an aging population. Curr Opin Microbiol 2016;29:63-7.

17. Kim MK, Bhattacharya J, Bhattacharya J. Is income inequality linked to infectious disease prevalence? A hypothesis-generating study using tuberculosis. Soc Sci Med 2024;345:116639.

18. Alividza V, Mariano V, Ahmad R, Charani E, Rawson TM, Holmes AH, et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect Dis Poverty 2018;7:76.


19. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 2018;41:513-21.

20. Korea Disease Control and Prevention Agency. National antimicrobial resistance surveillance in Korea 2023 annual report. https://www.kdca.go.kr/board/board.es?mid=a20310030000&bid=0132&act=view&list_no=726816&tag=&nPage=1 [Online] (last visited on 28 September 2025).
21. McGrath B, Broadhurst M, Roman C. Infectious disease considerations in immunocompromised patients. JAAPA 2020;33:16-25.

1. World Health Organization. Global tuberculosis report 2021. https://www.who.int/publications/i/item/9789240037021 [Online] (last visited on 28 September 2025).
2. Rodrigues C and Vadwai V. Tuberculosis: laboratory diagnosis. Clin Lab Med 2012;32:111-27.

3. Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 2018;67:e1-94.


4. National Healthcare Safety Network. CDC/NHSN Surveillance Definitions for Specific Types of Infections. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf [Online] (last visited on 28 September 2025).
5. Kim KH. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients. J Prev Med Public Health 2010;43:42-9.

6. Ministry of Health and Welfare. Senior general hospitals. https://www.mohw.go.kr/menu.es?mid=a10702030300 [Online] (last visited on 28 September 2025).
7. Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother 2018;50:67-100.


8. Kwak YG, Choi SH, Kim T, Park SY, Seo SH, Kim MB, et al. Clinical guidelines for the antibiotic treatment for community-acquired skin and soft tissue infection. Infect Chemother 2017;49:301-25.


9. Trajman A, Campbell JR, Kunor T, Ruslami R, Amanullah F, Behr MA, et al. Tuberculosis. Lancet 2025;405:850-66.

10. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration 2017;94:299-311.

11. Del Bono V and Giacobbe DR. Bloodstream infections in internal medicine. Virulence 2016;7:353-65.


12. Kaur R and Kaur R. Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad Med J 2021;97:803-12.

13. Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 2016;13:e1002119.


14. Dias SP, Brouwer MC, van de Beek D. Sex and gender differences in bacterial infections. Infect Immun 2022;90:e0028322.


15. Lee H, Kim J, Kim J, Park YJ, Jeong H, Kim H, et al. Tuberculosis notification status in the Republic of Korea, 2024. Public Health Wkly Rep 2025;18(Suppl 11):S6-S22.
16. Kline KA and Bowdish DM. Infection in an aging population. Curr Opin Microbiol 2016;29:63-7.

17. Kim MK, Bhattacharya J, Bhattacharya J. Is income inequality linked to infectious disease prevalence? A hypothesis-generating study using tuberculosis. Soc Sci Med 2024;345:116639.

18. Alividza V, Mariano V, Ahmad R, Charani E, Rawson TM, Holmes AH, et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect Dis Poverty 2018;7:76.


19. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 2018;41:513-21.

20. Korea Disease Control and Prevention Agency. National antimicrobial resistance surveillance in Korea 2023 annual report. https://www.kdca.go.kr/board/board.es?mid=a20310030000&bid=0132&act=view&list_no=726816&tag=&nPage=1 [Online] (last visited on 28 September 2025).
21. McGrath B, Broadhurst M, Roman C. Infectious disease considerations in immunocompromised patients. JAAPA 2020;33:16-25.
