Departments of Laboratory Medicine, Ajou University School of Medicine, Suwon, Korea
Corresponding to Wee Gyo Lee, E-mail: weegyo@ajou.ac.kr
Ann Clin Microbiol 2024;27(4):271-277. https://doi.org/10.5145/ACM.2024.27.4.7
Received on 2 October 2024, Revised on 3 November 2024, Accepted on 11 November 2024, Published on 3 December 2024.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Prototheca wickerhamii is an achlorophyllic algae that rarely acts as an opportunistic pathogen in humans. We report the case of a 66-year-old female patient with a history of diabetes mellitus who presented with a pus-like discharge from face, facial redness, and swelling. The patient was admitted to the emergency room with worsening facial pain. Gram staining from pus-like discharge revealed yeast like features; however, an isolated colony on a blood agar plate was not identified using VITEK 2 (Biomerieux) or matrix-assisted laser desorption/ionization time-of flight mass spectrometry (MALDI-TOF) using VITEK®MS (Biomerieux). Lactophenol cotton blue staining of the colony revealed characteristic sporangia and endospore features. After performing the protein extraction procedure for filamentous fungi (mold) isolates using ethanol and formic acid, MALDI-TOF MS identified the colony as Prototheca wickerhamii, with 99.9% confidence. This case indicates that Prototheca spp. require specialized sample preparation steps, such as the ethanol/formic acid extraction procedure, for identification using MALDI-TOF MS.
Matrix-assisted laser desorption-ionization mass spectrometry, Mycology, Protein, Prototheca
Prototheca is a genus of achlorophyllic green algae that rarely causes infections in humans however, is commonly found in the environment [1]. Among its species, Prototheca wickerhamii is the most frequently associated with human infections [2]. Prototheca infections in immunocompetent individuals are rare, and most cases occur in immunocompromised patients, such as those with diabetes and long-term steroid use [3]. In South Korea, 15 cases of Prototheca infection have been reported, of which 12 were caused by P. wickerhamii. In the other three cases, identification was achieved only at the genus level for two cases, and the other one was found to be caused by Prototheca zopfii [4].
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a technique that measures mass based on the time required for ionized particles to reach the detector in a vacuum. Recently, this technique has been applied in clinical microbiology for identification of microorganisms by analysis of their protein components. The introduction of MALDI-TOF MS in clinical microbiology has enabled the rapid and accurate identification of microorganisms. Among the 12 reported cases of P. wickerhamii in South Korea, only one used MALDI-TOF MS for identification; however, the detailed procedure was not described [5]. Therefore, we report a case of cutaneous infection by P. wickerhamii that was identified using MALDI-TOF MS, discuss considerations for identification, and detail a literature review.
Patient information: A 66-year-old female patient with a one-year history of facial abscesses and pain was admitted to the emergency room. The patient had been receiving antibiotic treatment at a local hospital but the symptoms worsened. The patient also had a history of diabetes, hypertension, and hyperlipidemia.
Clinical findings: Examination revealed swelling, severe tenderness, and a purulent discharge with erythema across the face; although, the vitals of the patient were stable and within normal limits.
Diagnostic assessment: Laboratory tests indicated a normal white blood cell count of 10.2×10⁹/L (neutrophil ratio of 77%), normal C-reactive protein level of 0.14 mg/L, and an elevated erythrocyte sedimentation rate of 32 mm/h.
On the second day of hospitalization, the patient underwent surgical abscess removal. Abscess cultures from both sets grew dry white colonies on blood agar (Fig. 1A). Gram staining revealed the presence of gram-positive microorganisms with a yeast-like appearance. The colonies were subcultured on Sabouraud dextrose agar, and white cream-like colonies grew on the second day of subculture. Lactophenol cotton blue staining revealed sporangia of varying sizes containing endospores (Fig. 1B). The yeast-like organisms were not identified using VITEK 2® (Biomerieux) YST card and MALDI-TOF MS performed with VITEK®MS (Biomerieux) (Fig. 2A). MALDI-TOF MS was performed using the yeast identification protocol, which uses formic acid and α-Cyano-4-hydroxycinnamic acid (CHCA) matrix. MALDI-TOF MS results were analyzed based on the VITEK®MS IVD database (version 3.2)
Considering that the characteristic endospores, sporangia structures, and the initial MALDI-TOF MS spectrum were insufficient for identification, we suspected that the thick cell wall might have hindered adequate protein extraction. Therefore, a mold-extraction protocol was performed using ethanol and formic acid. Subsequently, MALDI-TOF MS successfully identified the organism as P. wickerhamii with a confidence value of 99.9% (Fig. 2B). The organism was identified as P. wickerhamii when re-tested with the VITEK YST system.
The mold-extraction protocol was as follows: 70% ethanol was mixed with the colony, centrifuged for 2 min, and the supernatant was removed. Next, 40 µL of 70% formic acid and 40 µL of 100% acetonitrile were added, centrifuged for 2 min, and 1 µL of the supernatant was deposited on the MS target slide. After drying, 1 µL of CHCA matrix was added, the sample was given time to crystalize, and the slide was analyzed with MALDI-TOF MS following the standard protocol.
Therapeutic intervention: The patient was discharged after surgery with a prescription for oral amoxicillin/clavulanate 625 mg, to be taken three times daily for two weeks.
Follow-up and outcomes: After the patient’s discharge, P. wickerhamii was identified, and the clinical team was advised to use antifungal agents such as amphotericin B. However, the patient refused further treatment and was lost to follow-up. Therefore, it was not possible to assess the treatment response or continue monitoring.
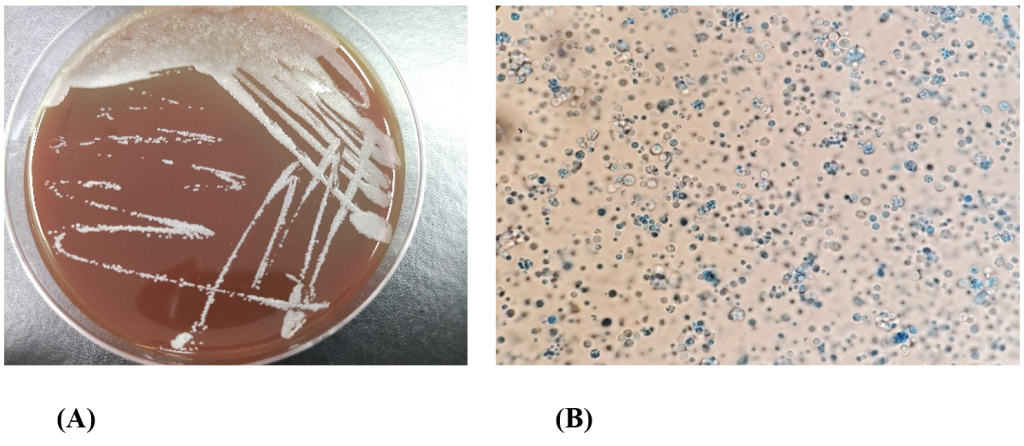
Fig. 1. Colony and microscopic morphology of Prototheca wickerhamii. (A) White dry colonies on BAP after 24 hours of incubation at 35℃ with 5% CO2. (B) Lactophenol cotton blue stain of P. wickerhamii showing endospore and sporangia features (oil immersion, × 1000 magnification). BAP, blood agar plate.
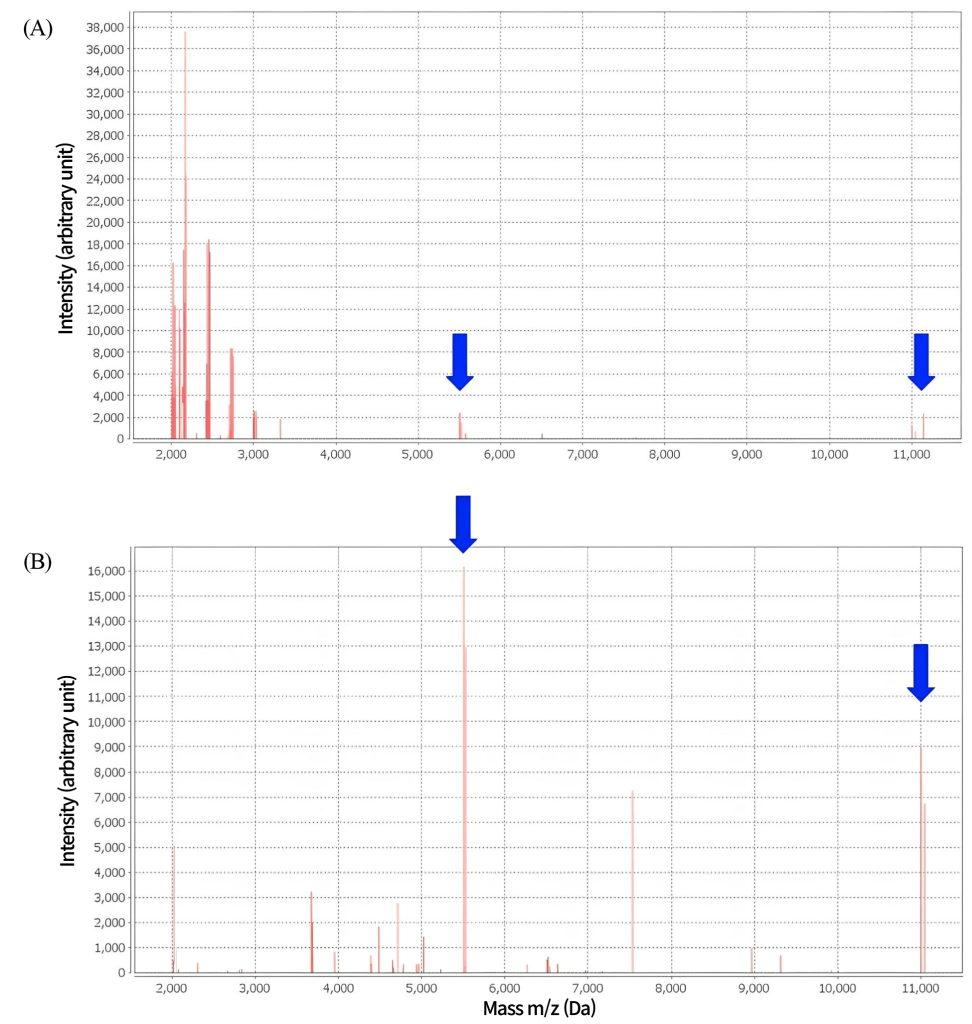
Fig. 2. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry Prototheca wickerhamii spectrum. (A) Spectrum of P. wickerhamii after yeast sample preparation with formic acid showing ambiguous identification. (B) Spectrum of P. wickerhamii after mold sample preparation using ethanol/formic acid extraction.
Prototheca species are non-photosynthetic green algae that resemble fungi by forming yeast-like colonies when cultured and are susceptible to antifungal agents. Although these species are usually environmental, some species, such as P. wickerhamii, can cause opportunistic infections [6]. Protothecosis can range from localized skin infections to systemic disease. Immunocompetent patients typically present with localized skin lesions and have a favorable prognosis, whereas immunocompromised patients can develop systemic infection [7].
In this case, the organism was identified using MALDI-TOF MS only after an additional protein extraction procedure was performed for the mold isolates. This was likely due to the thick walls of P. wickerhamii, inhibiting sufficient protein extraction necessary for spectrum analysis. We used ethanol and formic acid for additional protein extraction; however, ultrasonication can be used for P. wickerhamii protein extraction for MALDI-TOF MS, as noted by Murugaiyan et al. [8].
Although MALDI-TOF MS allows rapid and convenient microbial identification in clinical laboratories, it is limited in identifying rare organisms that are not included in the MALDI-TOF MS library. In the case reported by Fernández et al. [9], the organism was not identified using MALDI-TOF MS, even after an additional protein extraction step. Sequencing of the D1/D2 region of 26S rDNA identified the organism as Prototheca zopfii var. hydrocarbonea. The failure of identification using MALDI-TOF MS in this case was likely due to the absence of spectrum data in the MALDI-TOF MS library.
MALDI-TOF MS requires fresh colonies to be grown from cultures for protein extraction. Testing organisms that are difficult to culture, such as anaerobic and mycobacterial species, can be challenging. According to Bizzini et al. [10], among the 410 MALDI-TOF MS tests performed using the microflex LT (Bruker Daltonics), 133 cases (score < 1.7) were not correctly identified, primarily for two reasons: the absence of spectrum data in the library (58.6%) or failure to obtain sufficient spectrum data for analysis (41.4%).
Traditionally, Protothecosis were diagnosed using special staining of tissue samples. Periodic acid-Schiff, Grocott, or Gomori methenamine silver stains were used to visualize the characteristic endospores and sporangia structures of Prototheca species. Nowadays, with the advancement of automated equipment, VITEK-2 and MALDI-TOF MS have become the primary tools for identifying Prototheca species in laboratories. However, in this case, P. wickerhamii was not identified using VITEK-2 or the standard MALDI-TOF MS protocol, but was only identified after mold isolate-specific processing procedures followed by VITEK MS were used.
In our case, P. wickerhamii was not identified using VITEK YST, but was identified only upon retesting. Conversely, when retested with VITEK MS without a pre-procedure step, identification was still not achieved. While VITEK-2 is generally used for the diagnosis of Prototheca species, its failure may be due to colony freshness and turbidity. Therefore, when fungal colonies are not identified by standard methods that use automated equipment, it may be necessary to retest or apply different approaches. Similarly, in a report by Sardana et al. [11], P. wickerhamii was not identified using VITEK-2 or MALDI-TOF MS and was only identified after mold isolate pretreatment and subsequent MALDI-TOF MS analysis was performed. In addition, molecular diagnostics such as 16S rRNA and 18S rDNA sequencing can be used for accurate microbial identification.
As summarized in Table 1 [12–21], most reported cases of Prototheca infections in South Korea were identified using traditional methods, such as special staining with biopsy or cultured colonies, and visual confirmation of the characteristic sporangia and endospores. In the case reported by Kim et al. [5], VITEK MS was used for the diagnosis of P. wickerhamii, but this study did not discuss the pretreatment processes before MALDI-TOF MS. Although it is well known that adding formic acid to the protein extraction procedure improves the identification results for yeast using VITEK MS, standardized protocols for filamentous fungi are still lacking. This makes it challenging to accurately identify filamentous fungi using MALDI-TOF MS [22]. Here, we reported a case of cutaneous protothecosis in which P. wickerhamii was identified using MALDI-TOF MS after the procedure for filamentous fungi.
Table 1. Clinical summary of P. wickerhamii reported cases in South Korea
| Study | Age/sex | Year | Underlying | Diagnosis | Location |
|---|---|---|---|---|---|
| Yang et al. [12] | 80/F | 1996 | – | VITEK YBC | Forearm |
| Kim et al. [13] | 55/F | 1996 | Intralesional steroid injection | API20C | Ankle |
| Kim et al. [14] | 62/F | 1997 | Long term steroid, DM | API20C | Forearm |
| 45/F | 1997 | Long term steroid | API20C | Cheek | |
| Lee et al. [15] | 88/F | 1999 | – | API ID 32 C | Forearm |
| Choi et al. [16] | 66/F | 2002 | DM | VITEK YBC | Forearm |
| Cho et al. [17] | 63/F | 2002 | Long term steroid | VITEK YBC | Forearm |
| Jun et al. [18] | 64/M | 2003 | DM, Liver cirrhosis | VITEK YBC | Upper arm |
| Lee et al. [19] | 65/M | 2006 | DM, Iatrogenic cushing Sx | API20C | Forearm |
| Moon et al. [20] | 73/F | 2007 | Long term steroid | VITEK YBC | Forearm |
| Chae et al. [21] | 79/F | 2015 | – | VITEK 2 YST | Forearm |
| Kim et al. [5] | 71/F | 2017 | – | VITEK 2 YST, VITEK MS | Tenosynovitis |
| Present case | 66/F | 2024 | DM | VITEK 2 YST, VITEK MS | Face |
Abbreviations: F, female; M, male; DM, diabetes mellitus; Sx, symptoms.
The genus Prototheca is a type of green algae known to cause opportunistic infections in humans, particularly in individuals with underlying conditions such as immunosuppression or diabetes mellitus. In these patients, Prototheca can cause cutaneous and soft tissue infections and, in rare cases, disseminated systemic infection. Automated biochemical identification systems, such as VITEK-2, and MALDI-TOF MS systems, like VITEK MS, can be used to identify P. wickerhamii. However, for identification using MALDI-TOF MS, adequate protein extraction with ethanol/formic acid, through a pretreatment procedure typically used for filamentous fungi, is required. Here, we described a case of P. wickerhamii identification using MALDI-TOF MS with filamentous fungi pretreatment, along with a brief literature review.
The study was approved by the Institutional Review Board of Ajou University Hospital (IRB No. 2023-0995-001). The board exempted the obtainment of informed consent.
No potential conflicts of interest relevant to this article were reported.
None.
The datasets generated during the current study are available from the corresponding author upon request.
1. Pfaller MA, Warnock DW. Candida, cryptococcus, and other yeasts of medical importance. In: Murray PR, Baron EJ, et al., editors. Manual of clinical microbiology. 9th ed. Washington (DC): American Society for Microbiology Press; 2007:1779.
2. Hillesheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med 2011;135:941–4. 

3. Kano R. Emergence of fungal-like organisms: prototheca. Mycopathologia 2020;185:747–54.
4. Seok JY, Lee Y, Lee H, Yi SY, Oh HE, Song JS. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol 2013;47:575–8.


5. Kim JE, Oh TH, Lee KH, Shin JH, Jung SI. Successful treatment of protothecal tenosynovitis in an immunocompetent patient using amphotericin B deoxycholate. Infect Chemother 2017;49:293–6.


6. Todd JR, King JW, Oberle A, Matsumoto T, Odaka Y, Fowler M, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol 2012;50:673–89.

7. Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev 2007;20:230–42.


8. Murugaiyan J, Ahrholdt J, Kowbel V, Roesler U. Establishment of a matrix-assisted laser desorption ionization time-of-flight mass spectrometry database for rapid identification of infectious achlorophyllous green micro-algae of the genus Prototheca. Clin Microbiol Infect 2012;18:461–7.

9. Fernández NB, Taverna CG, Vivot M, Córdoba S, Paravano L. First bloodstream infection due to Prototheca zopfii var. hydrocarbonea in an immunocompromised patient. Med Mycol Case Rep 2019;24:9–12.


10. Bizzini A, Jaton K, Romo D, Bille J, Prod’hom G, Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry as an alternative to 16S rRNA gene sequencing for identification of difficult-to-identify bacterial strains. J Clin Microbiol 2011;49:693–6.


11. Sardana R, Butta H, Mendiratta L, Jasuja S, Xess I, Singh G, et al. The balancing universe: the story of human invasion and the primitive yet evolutionary prototheca. Ann Pathol Lab Med 2023;10:132–7.
12. Yang JK, Jang IG, Park YM, Kim TY, Kim HO, Kim CW. A case of cutaneous protothecosis. Ann Dermatol 1996;8:206–10.
13. Kim ST, Suh KS, Chae YS, Kim YJ. Successful treatment with fluconazole of protothecosis developing at the site of an intralesional corticosteroid injection. Br J Dermatol 1996;135:803–6.

14. Kim JA, Moon SE, Song KY. Two cases of cutaneous protothecosis: unique histopathological findings with crystal violet staining and the therapeutic effect of itraconazole. Ann Dermatol 1997;9:201–7.
15. Lee ES, Kim JH, Lee SN. A case of cutaneous protothecosis with severe pustules and ulceration. Kor J Med Mycol 1999;4:131–6.
16. Choi JH, Suh MK, Shin DJ, Suh JC, Yeum JS, Lee HC, et al. A case of cutaneous protothecosis. Korean J Dermatol 2002;40:1116–20.
17. Cho BK, Ham SH, Lee JY, Choi JH. Cutaneous protothecosis. Int J Dermatol 2002;41:304–6.

18. Jun JH, Lee JB, Kim SJ, Lee SC, Won YH. A case of cutaneous protothecosis. Korean J Med Mycol 2003;8:30–4.
19. Lee WS, Kim YJ, Kim SY, Kim KM. A case of cutaneous protothecosis. Korean J Dermatol 2006;44:648–51.
20. Moon HS, Lee HK, Park K, Chae JD, Son SJ. A case of cutaneous protothecosis. Korean J Med Mycol 2007;12:70–4.
21. Chae SY, Lee KC, Lee HS, Jang YH, Lee S, Kim DW, et al. A case of cutaneous protothecosis. Korean J Med Mycol 2015;20:13–8.
22. Croxatto A, Prod’hom G, Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev 2012;36:380–407.

1. Pfaller MA and Warnock DW. Candida, cryptococcus, and other yeasts of medical importance. In: Murray PR, Baron EJ, et al. eds. Manual of clinical microbiology. 9th ed. Washington DC: American Society for Microbiology Press; 2007:1779.
2. Hillesheim PB and Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med 2011;135:941-4.
3. Kano R. Emergence of fungal-like organisms: prototheca. Mycopathologia 2020;185:747-54.
4. Seok JY, Lee Y, Lee H, Yi SY, Oh HE, Song JS. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol 2013;47:575-8.
5. Kim JE, Oh TH, Lee KH, Shin JH, Jung SI. Successful treatment of protothecal tenosynovitis in an immunocompetent patient using amphotericin B deoxycholate. Infect Chemother 2017;49:293-6.
6. Todd JR, King JW, Oberle A, Matsumoto T, Odaka Y, Fowler M, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol 2012;50:673-89.
7. Lass-Flörl C and Mayr A. Human protothecosis. Clin Microbiol Rev 2007;20:230-42.
8. Murugaiyan J, Ahrholdt J, Kowbel V, Roesler U. Establishment of a matrix-assisted laser desorption ionization time-of-flight mass spectrometry database for rapid identification of infectious achlorophyllous green micro-algae of the genus Prototheca. Clin Microbiol Infect 2012;18:461-7.
9. Fernández NB, Taverna CG, Vivot M, Córdoba S, Paravano L. First bloodstream infection due to Prototheca zopfii var. hydrocarbonea in an immunocompromised patient. Med Mycol Case Rep 2019;24:9-12.
10. Bizzini A, Jaton K, Romo D, Bille J, Prod’hom G, Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry as an alternative to 16S rRNA gene sequencing for identification of difficult-to-identify bacterial strains. J Clin Microbiol 2011;49:693-6.
11. Sardana R, Butta H, Mendiratta L, Jasuja S, Xess I, Singh G, et al. The balancing universe: the story of human invasion and the primitive yet evolutionary prototheca. Ann Pathol Lab Med 2023;10:132-7.
12. Yang JK, Jang IG, Park YM, Kim TY, Kim HO, Kim CW. A case of cutaneous protothecosis. Ann Dermatol 1996;8:206-10.
13. Kim ST, Suh KS, Chae YS, Kim YJ. Successful treatment with fluconazole of protothecosis developing at the site of an intralesional corticosteroid injection. Br J Dermatol 1996;135:803-6.
14. Kim JA, Moon SE, Song KY. Two cases of cutaneous protothecosis: unique histopathological findings with crystal violet staining and the therapeutic effect of itraconazole. Ann Dermatol 1997;9:201-7.
15. Lee ES, Kim JH, Lee SN. A case of cutaneous protothecosis with severe pustules and ulceration. Kor J Med Mycol 1999;4:131-6.
16. Choi JH, Suh MK, Shin DJ, Suh JC, Yeum JS, Lee HC, et al. A case of cutaneous protothecosis. Korean J Dermatol 2002;40:1116-20.
17. Cho BK, Ham SH, Lee JY, Choi JH. Cutaneous protothecosis. Int J Dermatol 2002;41:304-6.
18. Jun JH, Lee JB, Kim SJ, Lee SC, Won YH. A case of cutaneous protothecosis. Korean J Med Mycol 2003;8:30-4.
19. Lee WS, Kim YJ, Kim SY, Kim KM. A case of cutaneous protothecosis. Korean J Dermatol 2006; 44:648-51.
20. Moon HS, Lee HK, Park K, Chae JD, Son SJ. A case of cutaneous protothecosis. Korean J Med Mycol 2007;12:70-4.
21. Chae SY, Lee KC, Lee HS, Jang YH, Lee S, Kim DW, et al. A case of cutaneous protothecosis. Korean J Med Mycol 2015;20:13-8.
22. Croxatto A, Prod’hom G, Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev 2012;36:380-407.