1Department of Laboratory Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
2Department of Laboratory Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
3Department of Laboratory Medicine, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea
4Department of Laboratory Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea
5Department of Laboratory Medicine, Eunpyeong St. Mary’s Hospital, Catholic University College of Medicine, Seoul, Korea
6Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
Correspondence to Heungsup Sung, E-mail: sung@amc.seoul.kr
Ann Clin Microbiol 2025;28(1):5. https://doi.org/10.5145/ACM.2025.28.1.5
Received on 28 February 2024, Revised on 10 March 2025, Accepted on 11 March 2025, Published on 20 March 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Background: The coronavirus disease 2019 (COVID-19) pandemic has significantly impacted global infrastructure. We surveyed laboratories to analyze the changes in testing methods and procedures to improve future pandemic preparedness.
Methods: This study surveyed laboratory physicians and technologists in South Korea and analyzed responses from 126 of 323 institutions. The survey was conducted in May 2023 using the proficiency test of the Korean Association of External Quality Assessment Service and examined the diagnostic procedures, personnel, equipment, and quality control. The survey comprised 15 questions covering respondent demographics, public-private proficiency projects, COVID-19 testing procedures, and laboratory status.
Results: Of the 126 laboratories, 66.7% performed bacterial smear and culture, 65.9% had biosafety level 2 facilities, and 39.7% had separate nucleic acid extraction areas. Furthermore, 98.4% of the laboratories had biological safety cabinets, the median number of PCR machines was four units, and 77.8% had autoclaves. The median numbers of personnel managing and conducting tests were one and three, respectively. Additionally, 88.1% of the laboratories found the COVID-19 proficiency test helpful, with key benefits in terms of accuracy and skill improvement. COVID-19 tests were primarily used for symptomatic or contact person testing, pre-admission screening, and periodic proactive testing. Specialized testing laboratories conducted up to 50,000 tests daily, and tertiary hospitals conducted up to 1,500 tests. Emergency, pooled, and rapid antigen tests were widely used. Most respondents wanted future tests for respiratory viruses, bacteria, and viral diarrhea, indicating a willingness to participate.
Conclusion: Aggressive testing and collaboration between health agencies and laboratories are crucial for managing emerging diseases. Systematic preparations are essential to maintain and strengthen laboratory capabilities for future infectious disease outbreaks.
COVID-19, Outbreak response, Pandemics, PCR, SARS-CoV-2
The coronavirus disease 2019 (COVID-19) pandemic, declared by the World Health Organization on March 11, 2020, is the largest infectious disease of the 21st century and has significantly affected global infrastructure [1]. Unlike previous outbreaks such as severe acute respiratory syndrome (SARS), influenza A(H1N1)pdm09 virus, and Middle East respiratory syndrome (MERS), COVID-19 spread rapidly and globally from Wuhan, China, in late 2019. South Korea reported approximately 34 million confirmed cases, with total infections possibly closer to 45 million, considering undiagnosed cases [2].
The speed and accuracy of early testing for new infectious disease outbreaks are crucial [3]. Active testing, tracking, isolation, and treatment are essential to control the spread and prevent the collapse of the medical system until treatments and vaccines are developed. In early February 2020, the Korean Society for Laboratory Medicine (KSLM) collaborated closely with the Korea Centers for Disease Control and Prevention (now the Korea Disease Control and Prevention Agency [KDCA]) to help polymerase chain reaction (PCR)-based molecular diagnostic kits quickly receive emergency use authorization [4,5]. This facilitated the expansion of COVID-19 testing to private laboratories through nationwide proficiency testing, effectively controlling its initial spread. Conversely, several countries with an inadequate supply of diagnostic kits experienced significant disruptions as they failed to prevent the spread of infection [6].
From January 2020 to June 2023, spanning the first to seventh waves of outbreaks, the introduction of vaccines and changes in quarantine measures significantly altered the trajectory of the pandemic. New testing methods, reagent approvals, and testing reimbursement standards have been introduced, leading to changes in the testing methods, equipment, and personnel in clinical microbiology laboratories.
As the scale and severity of COVID-19 outbreaks have recently decreased, significant changes have been introduced in the utilization of testing methods and individual test procedures. To prepare for the continued spread of COVID-19 and the potential emergence of new pandemics, we aimed to collect and analyze the COVID-19 response experiences and achievements of clinical microbiology laboratories through a survey.
This was a cross-sectional survey study.
This study analyzed survey results of laboratory physicians and technologists working in medical institutions and referral laboratories in South Korea. From May 23 to 26, 2023, in conjunction with the “viral molecular testing II (COVID-19 molecular diagnostic test) proficiency test” conducted by the Korean Association of External Quality Assurance Service (KEQAS), the survey examined infectious disease diagnostic testing items, personnel, equipment, and quality control.
The survey was sent to all 323 institutions participating in COVID-19 molecular diagnostics. Responses were collected via Google Forms after the participant of each institution reviewed the study topic and survey explanation online.
The survey consisted of 15 questions. The first two questions gathered basic information regarding the respondents, such as their affiliated institutions and job positions. Three questions focused on the publicprivate integrated proficiency evaluation project, addressing items related to ongoing infectious disease proficiency evaluation projects, potential future additions, and the willingness of the respondents to participate. Seven questions focused on COVID-19 testing and proficiency assessment, addressing aspects such as quality control, testing purpose, emergency, pooled, and antigen testing, along with maximum daily testing capacity. Finally, six questions examined laboratory status, including the types of tests, facilities, and workforce, to evaluate the ability to respond to future emerging infectious diseases.
Because the survey was administered to all laboratories performing COVID-19 molecular diagnostic tests, selection bias is minimal. However, we did not include laboratories that perform only non-molecular diagnostic tests, such as antigen tests for COVID-19.
The study size was not estimated since this was a descriptive study.
The data were organized and analyzed using Microsoft Excel to identify the major response trends through frequency analysis.
Target institutions included 291 private laboratories that participated in the viral molecular testing II proficiency test and 32 public laboratories that participated in special projects commissioned by the KDCA. Finally, 126 laboratories (response rate of 39.0%) responded.
The organizations of the respondents included general hospitals (61.1%, 77/126), tertiary hospitals (15.9%, 20/126), and public laboratories (11.9%, 15/126). Among the 15 respondents from public laboratories, eight were affiliated with public medical institutions, such as tertiary hospitals of national universities, regional medical centers, the Korea Institute of Radiological and Medical Sciences, and the National Health Insurance Service Ilsan Hospital. The remaining seven were comprised of the KDCA and each provincial Institute of Health and Environment (Table 1). Most respondents were medical laboratory technologists (116/126, 92.1%), with one (0.8%) serving as a laboratory physician.
Table 1. Distribution of respondents based on affiliated institutions and positions
| Category | No. of respondents (%) |
|---|---|
| Affiliated institution | |
| General hospital | 79 (62.7) |
| Tertiary hospital | 20 (15.9) |
| Public laboratory | 15 (11.9) |
| Specialized testing laboratory | 11 (8.7) |
| Primary clinic | 1 (0.8) |
| Job position | |
| Medical laboratory technologists | 116 (92.0) |
| Public health researcher | 7 (5.6) |
| Specialized research fellow | 2 (1.6) |
| Laboratory physician | 1 (0.8) |
| Total | 126 (100.0) |
Of the 126 responding laboratories, 84 (66.7%) conducted bacterial smears and cultures, 76 (60.3%) conducted acid-fast bacillus smears, and 53 (42.1%) performed acid-fast bacillus cultures. Sixty-five (51.6%) performed microbial molecular testing, excluding hepatitis or human immunodeficiency virus (HIV). Eightythree (65.9%) laboratories had a biosafety level 2 (BSL2) laboratory. Fifty (39.7%) laboratories maintained separate areas for nucleic acid extraction, reagent preparation, and nucleic acid amplification. Fifty-one (40.5%) laboratories had a negative-pressure nucleic acid extraction room within their molecular diagnostic area. Twenty-five (19.8%) laboratories had a negative-pressure tuberculosis testing room, whereas 11 (8.7%) had a tuberculosis testing room with a double-barrier anteroom. Six (4.8%) laboratories did not have access to these facilities (Table 2).
Table 2. Distribution of laboratory capabilities and facilities among responding laboratories for various testing methods
| Category | No. of responses (%) |
|---|---|
| Microbiological tests performed | |
| Serologic test for HIV | 95 (75.4) |
| Serologic test for hepatitis | 90 (71.4) |
| Bacterial smear and culture | 84 (66.7) |
| Acid-fast bacillus smear | 76 (60.3) |
| Microbial molecular test (excluding hepatitis or HIV) | 65 (51.6) |
| Serologic test for infectious disease (excluding hepatitis) | 65 (51.6) |
| Parasitic testing | 60 (47.6) |
| Fungal smear and culture | 54 (42.9) |
| Acid-fast bacillus culture | 53 (42.1) |
| Molecular test for hepatitis | 49 (38.9) |
| Microbial rapid antigen test (using immunochromatography, excluding hepatitis or HIV) | 44 (34.9) |
| Microbial antigen test (using immunoassay, excluding hepatitis or HIV) | 30 (23.8) |
| Molecular test for HIV | 16 (12.7) |
| Facility status | |
| Biosafety level 2 laboratory (Separate space, biological hazard sign, autoclave, biosafety cabinet, etc.) | 83 (65.9) |
| Negative pressure nucleic acid extraction room | 51 (40.5) |
| Molecular testing laboratory separated into three areas (nucleic acid extraction, reagent preparation, nucleic acid amplification) | 50 (39.7) |
| Negative pressure tuberculosis testing room | 25 (19.8) |
| Tuberculosis testing room with double barrier anteroom | 11 (8.7) |
| None of the above | 6 (4.8) |
| Total | 126 (100.0) |
Abbreviation: HIV, human immunodeficiency virus.
Most laboratories (98.4%, 124/126) had biological safety cabinets, with a median of two units. Ninety-two (73.0%) laboratories had more than two units. For the molecular testing of infectious diseases, the median number of PCR machines was two units, with 87 (73.1%) having more than two units. In tertiary hospitals, the median number of PCR machines was four, while specialized testing laboratories had a median of 18.5 devices. Ninety-eight (77.8%) laboratories had an autoclave for biological waste disposal, with a median of one unit. Nineteen (15.1%) laboratories had two or more autoclaves (Fig. 1).
The median number of personnel managing infectious disease diagnostic testing (laboratory physicians or public health research officers) was one, with 37 (29.4%) laboratories having two or more personnel. The median number of personnel conducting infectious disease diagnostic testing was three, with 47 (37.3%) having five or more personnel (Fig. 2).
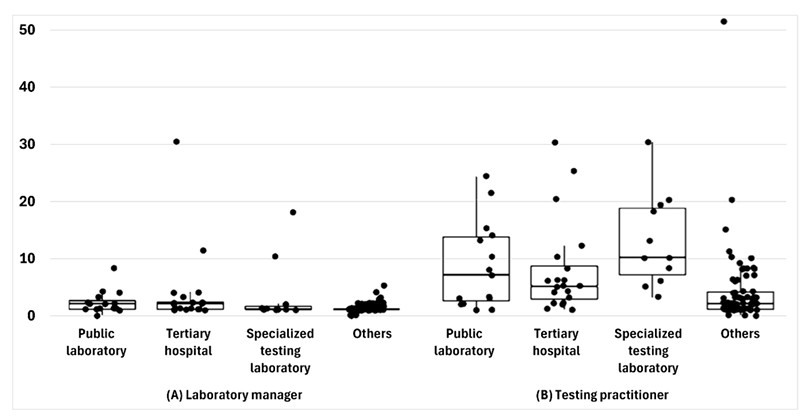
Fig. 2. Personnel conducting diagnostic testing for infectious diseases, categorized by type of affiliated institution.
In response to the question on the effectiveness of the COVID-19 proficiency test, 111 (88.1%) laboratories reported that the biannual test was significantly helpful, with a score of four or five out of five. The most beneficial aspects were assessing the accuracy through periodic external quality control (115/126, 91.3%), improving the skills of testing practitioners and supervisors (67/126, 53.2%), and obtaining positive specimens for quality control during the early stages of COVID-19 (33/126, 26.2%). Suggested areas for improvement included increasing sample volume (66/126, 52.4%), improving data entry (28/126, 22.2%), and enhancing transport and packaging (24/126, 19.0%).
COVID-19 molecular tests were mainly used for symptomatic individuals and those who had come in contact with (n = 112), pre-admission screening of all patients (n = 99), and pre-admission screening of highrisk patients (n = 93) (Table 3). Specialized testing laboratories perform up to 50,000 tests daily, whereas tertiary hospitals perform up to 1,500 tests daily (Fig. 3). The median maximum number of tests per day was 825.5, 396, and 317.5 in specialized testing laboratories, public laboratories, and tertiary hospitals, respectively. While public and specialized testing laboratories were more likely to test symptomatic patients and contact persons, hospitals, including tertiary hospitals, tested for various purposes, including international travel testing and pre-admission screening.
Table 3. Usage of coronavirus disease 2019 molecular test by testing purpose
| Public laboratories (n = 15) | Tertiary hospitals (n = 20) | Specialized testing laboratories (n = 11) | Other (n = 80) | Total | |
|---|---|---|---|---|---|
| Symptomatic and contact person testing | 13 | 18 | 10 | 71 | 112 |
| Pre-admission screening of all patients | 9 | 16 | 4 | 70 | 99 |
| Pre-admission screening of high-risk patients | 9 | 15 | 4 | 65 | 93 |
| International travel testing | 6 | 7 | 5 | 51 | 69 |
| Proactive testing in a geriatric hospital | 9 | 7 | 6 | 42 | 64 |
| Periodic proactive testing | 4 | 13 | 5 | 41 | 63 |
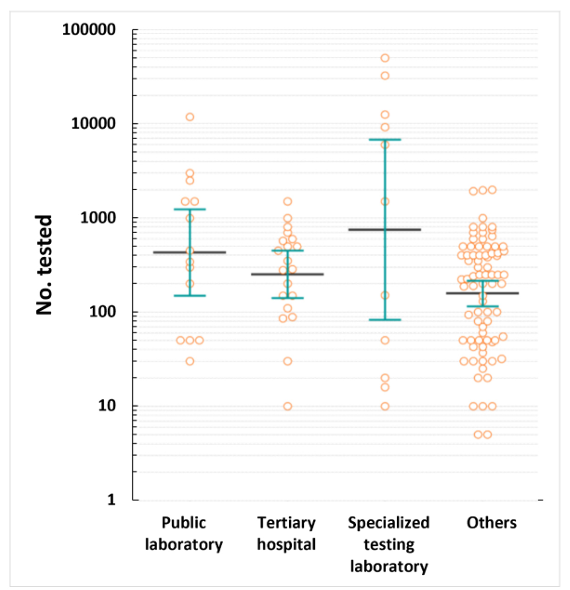
Fig. 3. Maximum number of coronavirus disease 2019 molecular diagnostic tests per day.
COVID-19 emergency testing was used evenly for the pre-admission screening of high-risk patients (n = 85), symptomatic and contact person testing (n = 84), and pre-admission screening of all patients (n = 80) (Table 4). In contrast, pooled testing was primarily used for pre-admission screening of all patients (n = 99), high-risk patients (n = 96), periodic proactive testing (n = 47), and proactive testing in geriatric hospitals (n = 43) (Table 5). Professional rapid antigen tests were mainly used for symptomatic and contact person testing (n = 84), pre-admission screening of all patients (n = 70), and pre-admission screening of caregivers (n = 65) (Table 6).
Table 4. Usage of coronavirus disease 2019 emergency test by testing purpose
| Public laboratories (n = 15) | Tertiary hospitals (n = 20) | Specialized testing laboratory (n = 11) | Other (n = 80) | Total | |
|---|---|---|---|---|---|
| Pre-admission screening of high-risk patients | 10 | 15 | 7 | 53 | 85 |
| Symptomatic and contact person testing | 12 | 13 | 8 | 51 | 84 |
| Pre-admission screening of all patients | 9 | 13 | 7 | 51 | 80 |
| Periodic proactive testing | 6 | 9 | 6 | 28 | 49 |
| International travel testing | 4 | 5 | 8 | 22 | 39 |
| Proactive testing in a geriatric hospital | 7 | 4 | 6 | 15 | 32 |
Table 5. Usage of coronavirus disease 2019 pooling test by testing purpose
| Public laboratories (n = 15) | Tertiary hospitals (n = 20) | Specialized testing laboratory (n = 11) | Other (n = 80) | Total | |
|---|---|---|---|---|---|
| Pre-admission screening of all patients | 10 | 15 | 9 | 65 | 99 |
| Pre-admission screening of high-risk patients | 8 | 18 | 10 | 60 | 96 |
| Periodic proactive testing | 5 | 9 | 9 | 24 | 47 |
| Proactive testing in a geriatric hospital | 5 | 4 | 10 | 24 | 43 |
| Military enlistment-related testing | 4 | 2 | 9 | 7 | 22 |
| Trainee screening | – | – | – | 2 | 2 |
| Genetic variant analysis | 1 | – | – | – | 1 |
| New employee screening | – | – | – | 1 | 1 |
Table 6. Usage of coronavirus disease 2019 professional rapid antigen test by testing purpose
| Public laboratories (n = 15) | Tertiary hospitals (n = 20) | Specialized testing laboratory (n = 11) | Other (n = 80) | Total | |
|---|---|---|---|---|---|
| Symptomatic and contact person testing | 12 | 8 | 2 | 62 | 84 |
| Pre-admission screening of all patients | 7 | 6 | 1 | 56 | 70 |
| Pre-admission screening of caregivers | 6 | 6 | 2 | 51 | 65 |
| Pre-admission screening of high-risk patients | 8 | 10 | 2 | 40 | 60 |
| Periodic proactive testing | 2 | 2 | 1 | 27 | 32 |
| Proactive testing in a geriatric hospital | 4 | 2 | – | 16 | 22 |
| Employee surveillance | – | – | – | 2 | 2 |
| International travel testing | – | 2 | – | – | 2 |
| Outpatient testing | 1 | – | – | 1 | 2 |
All respondent laboratories participated in COVID-19 molecular testing in the infectious disease proficiency test conducted as special projects by the KDCA and KEQAS. Additionally, 44 and 31 laboratories participated in HIV-1 and syphilis testing, respectively. Regarding future proficiency test items that respondents believed needed to be included to improve the standardization and accuracy of infectious disease diagnostics, molecular testing for other respiratory viruses such as metapneumoviruses, seasonal coronaviruses (OC43, HKU1, NL63, and 229E), rhinoviruses, and enteroviruses was the most commonly selected (n = 56), followed by bacterial culture/molecular testing for diarrhea (n = 21) and viral antigen/ molecular testing for diarrhea (n = 19). Seventy-six laboratories indicated that they would be willing to participate in the proficiency test if other respiratory viral molecular diagnostic tests were included.
Most respondents were medical laboratory technologists (92.1%). Of the 126 responding laboratories, 66.7% conducted bacterial smears/cultures, 60.3% performed acid-fast bacillus smears, and 65.9% had BSL2 labs. COVID-19 testing was mainly for symptomatic individuals, pre-admission screening, and highrisk patients, with specialized labs performing up to 50,000 tests daily. The COVID-19 proficiency test was deemed highly effective (88.1%), aiding accuracy and skill improvement. Future improvements suggested included expanding sample volume and enhancing data entry and transport protocols. Respondents supported adding molecular tests for other respiratory viruses to proficiency evaluations.
South Korea was the fastest country in the world to authorize COVID-19 diagnostic test products for emergency use, allowing private laboratories to participate in early diagnostic testing [7]. This approach has been recognized as an exemplary case for enhancing national diagnostic capabilities and preventing the spread of COVID-19 [6]. This swift response was based on the experiences of the KSLM in responding to the MERS outbreak in 2015 and the Zika virus outbreak in 2016 as well as the proficiency test conducted by the KEQAS. As COVID-19 is difficult to diagnose based on clinical symptoms alone, laboratory tests for the causative virus are crucial [8]. Strategies for setting appropriate test objectives and selecting appropriate test methods are essential and should be flexible enough to adapt to changes in community epidemiology, infection reproduction index, and testing capacity.
This study evaluated the COVID-19 response experiences of medical institutions in South Korea and their laboratory capabilities for future infectious disease outbreaks. Based on initial COVID-19 responses, laboratories must complete testing for suspected and emergency patients within 6–12 hours, operate 2–3 times daily, and perform bacterial culture, antimicrobial susceptibility testing, and acid-fast bacillus smears. Having sufficiently skilled personnel is crucial for effectively managing these tasks and ensuring preparedness for emerging and re-emerging pandemics [9,10].
Additionally, the molecular diagnostics environment requires a separate space for the pretreatment of specimens (including nucleic acid extraction), reagent preparation, and nucleic acid amplification. Laboratories should possess at least two PCR devices to prepare for any breakdowns. Facilities and equipment with BSL2 are essential, and setting up a specimen collection space will benefit from the operational experience of previous COVID-19 screening clinics [11,12]. According to this study, 65.9% of the laboratories have BSL2 facilities, and 39.7% have separate spaces for nucleic acid extraction, including negative-pressure nucleic acid extraction rooms. This indicates that the competencies necessary to respond to emerging infectious diseases can be met by further enhancing these capabilities. Cooperation with specialized testing laboratories is essential for processing large numbers of specimens, and a more systematic infectious disease response can be achieved by utilizing the proficiency test program of the KEQAS.
Satisfaction with the COVID-19 proficiency evaluation project was very high (88.1%), contributing to improvements in the accuracy of tests and the proficiency of practitioners through periodic external quality control. Based on these experiences, it is expected that the laboratory infrastructure suitable for responding to emerging infectious diseases can be expanded by managing national laboratory testing capabilities and implementing appropriate certifications when necessary.
Molecular testing is the standard method for COVID-19 diagnosis and involves amplifying the nucleic acids of SARS-CoV-2 from a patient’s specimen to detect genes. Owing to its high sensitivity and specificity, real-time reverse transcriptase PCR (RT-PCR) tests have been used for COVID-19 confirmation and screening in most laboratories in South Korea. These tests have been performed for various purposes, including international travel testing and pre-admission screening, as well as for symptomatic patients. South Korea’s molecular diagnostic capabilities rely primarily on real-time RT-PCR; however, real-time RT-PCR has limitations in that a positive result does not accurately reflect infectivity. It also requires large reagent and consumable requirements, takes more than three hours to obtain test results, and is relatively expensive, creating a bottleneck in diagnosis, tracking, and isolation strategies in the early stages of the pandemic. The KSLM’s call for the Ministry of Food and Drug Safety to approve large-capacity automated testing systems during the fourth wave of COVID-19 in July 2021 is considered an appropriate measure to address this situation [6].
COVID-19 emergency tests are mainly used for pre-admission screening of high-risk patients, symptomatic individuals, or close contacts [13]. In the U.S., cartridge-type PCR tests that can be used with GeneXpert systems (Cepheid), which provides results within approximately 1 hour, were introduced early. In South Korea, GeneXpert was also primarily used for the pre-admission screening of high-risk patients [14]. This equipment, which is automated from nucleic acid extraction to PCR, allows tests to be conducted by non-specialists. However, it has lower sensitivity and may produce false-negative results in patients with low viral loads [15]. Additionally, the high price of test kits and the reimbursement criteria of the national insurance system, which only covers tests for patients requiring emergency surgery or those in critical condition, pose significant challenges.
Pooled testing was primarily used for pre-admission screening and contributed to meeting the explosive demand for tests during the third wave of COVID-19 in South Korea [16]. Pooled testing can increase testing capacity while maintaining the same duration and resources required for testing; however, it becomes less efficient as prevalence rates increase [17]. The European Center for Disease Prevention and Control recommends its use up to a positivity rate of 5%; however, its utility diminishes when the positivity rates exceed 1% [18]. The dilution of specimens during pooling increases the likelihood of false-negative results; therefore, it should be used cautiously, considering the potential need for additional testing owing to decreased test sensitivity.
Professional rapid antigen tests have lower sensitivity than PCR tests and can produce false positive results due to cross-reactions with other respiratory viruses [19]. However, they have high specificity, allowing for the quick identification of infected individuals, with test results available within 15–30 minutes and lower test costs. This study found that rapid antigen tests were used for symptomatic and contact person testing and pre-admission screening of patients and caregivers. The clinical sensitivity of the authorized antigen tests in South Korea is 41.5%, indicating a high false-negative rate in cases of low viral load, necessitating retesting [20].
The study surveyed 323 COVID-19 molecular testing laboratories; however, the response rate was low, with only approximately 40% of the laboratories participating. The accuracy of some responses may be questionable as most respondents were testing practitioners, not laboratory managers, and may have relied on memory as the demand for COVID-19 testing decreased. Further investigations using KSLM and KEQAS are necessary to obtain a more objective and accurate assessment of the status of clinical laboratories in preparation for new infectious disease pandemics.
This study aimed to collect and analyze the experiences and achievements of clinical microbiology laboratories in responding to COVID-19 through a survey. The findings can also be applicable in preparing for the emergence of future pandemics.
Clinical laboratories are primarily responsible for testing specimens to support clinical decision-making. In the early stages of an emerging and re-emerging infectious disease outbreak, aggressive testing of suspected patients and contacts in the absence of vaccines and treatment drugs, as well as patient tracing, triage, isolation, and treatment, are essential. As early experience with COVID-19 has shown, testing capacity is a critical factor in determining the scale of an outbreak. Expanding the initial testing capacity for emerging and re-emerging infectious diseases requires close collaboration between the KDCA, Ministry of Food and Drug Safety, Regional Institute of Health and Environment, reagent companies, private clinical laboratories, and relevant academic societies.
A survey of clinical laboratories in South Korea showed that many clinical laboratories have the personnel and facilities to expand their diagnostic capabilities and respond to emerging infectious diseases in a short period. Continuous updates on proficiency testing and training reinforcement for laboratory personnel are recommended to sustain laboratory readiness for future outbreaks.
This was not a human population study; therefore, approval by the institutional review board and informed consent were not required.
Jung-Hyun Byun has been an associate editor of the Annals of Clinical Microbiology since 2022. However, she was not involved in the review process of this article. No other potential conflicts of interest relevant to this article were reported.
This study was supported by the Research Fund (2023) of the Korean Society of Clinical Microbiology.
The data sets generated during this study are available from the corresponding author upon request.
The authors sincerely thank all the respondents for the questionnaires.
1. Chen J, Vullikanti A, Santos J, Venkatramanan S, Hoops S, Mortveit H, et al. Epidemiological and economic impact of COVID-19 in the US. Sci Rep 2021;11:20451.


2. Lee C, Apio C, Park T. Estimation of undetected asymptomatic COVID-19 cases in South Korea using a probabilistic model. Int J Environ Res Public Health 2021;18:4946.


3. Yimer SA, Booij BB, Tobert G, Hebbeler A, Oloo P, Brangel P, et al. Rapid diagnostic test: a critical need for outbreak preparedness and response for high priority pathogens. BMJ Glob Health 2024;9:e014386.


4. Sung H, Yoo CK, Han MG, Lee SW, Lee H, Chun S, et al. Preparedness and rapid implementation of external quality assessment helped quickly increase COVID-19 testing capacity in the Republic of Korea. Clin Chem 2020;66:979-81.


5. Kim YJ, Sung H, Ki CS, Hur M. COVID-19 testing in South Korea: current status and the need for faster diagnostics. Ann Lab Med 2020;40:349-50.


6. Olalekan A, Iwalokun B, Akinloye OM, Popoola O, Samuel TA, Akinloye O. COVID-19 rapid diagnostic test could contain transmission in low- and middle-income countries. Afr J Lab Med 2020;9:1255.


7. Huh HJ, Hong KH, Kim TS, Song SH, Roh KH, Lee H, et al. Surveillance of coronavirus disease 2019 (COVID-19) testing in clinical laboratories in Korea. Ann Lab Med 2021;41:225-9.


8. Zayed RA, Omran D, Zayed AA. COVID-19 clinical and laboratory diagnosis overview. J Egypt Public Health Assoc 2021;96:25.


9. Reidy N, Coetzee H, Roche C, Brazil E, O’Sullivan L, Brady D, et al. SARS-CoV-2 testing and patient waiting times in the emergency department. Ir Med J 2022;115:633.
10. Jarrett M, Garrick R, Gaeta A, Lombardi D, Mayo R, McNulty P, et al. Pandemic preparedness: COVID-19 lessons learned in New York’s hospitals. Jt Comm J Qual Patient Saf 2022;48:475-91.


11. Kaufer AM, Theis T, Lau KA, Gray JL, Rawlinson WD. Laboratory biosafety measures involving SARS-CoV-2 and the classification as a Risk Group 3 biological agent. Pathology 2020;52:790-5.


12. Naeem W, Zeb H, Rashid MI. Laboratory biosafety measures of SARS-CoV-2 at containment level 2 with particular reference to its more infective variants. Biosaf Health 2022;4:11-4.


13. Lee K. Laboratory diagnosis of COVID-19 in Korea. Ewha Med J 2021;44:1-10.
14. Hong KH, Kim GJ, Roh KH, Sung H, Lee J, Kim SY, et al. Update of guidelines for laboratory diagnosis of COVID-19 in Korea. Ann Lab Med 2022;42:391-7.


15. Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2020;8:CD013705.


16. Kim SY, Lee J, Sung H, Lee H, Han MG, Yoo CK, et al. Pooling upper respiratory specimens for rapid mass screening of COVID-19 by real-time RT-PCR. Emerg Infect Dis 2020;26:246972.


17. Centers for Disease Control and Prevention. Interim guidance for use of pooling procedures in SARS-CoV-2 diagnostic, screening, and surveillance testing. https://stacks.cdc.gov/view/cdc/90937 [Online] (last visited on 28 February 2025).
18. European Centre for Disease Prevention and Control (ECDC). Methodology for estimating point prevalence of SARS-CoV-2 infection by pooled RT-PCR testing. https://www.ecdc.europa.eu/en/publications-data/methodology-estimating-point-prevalence-sars-cov-2-infection-pooled-rt-pcr [Online] (last visited on 28 May 2020).
19. Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol 2020;129:104455.


20. Lee J, Kim SY, Huh HJ, Kim N, Sung H, Lee H, et al. Clinical performance of the Standard Q COVID-19 rapid antigen test and simulation of its real-world application in Korea. Ann Lab Med 2021;41:588-92.


1. Chen J, Vullikanti A, Santos J, Venkatramanan S, Hoops S, Mortveit H, et al. Epidemiological and economic impact of COVID-19 in the US. Sci Rep 2021;11:20451.
2. Lee C, Apio C, Park T. Estimation of undetected asymptomatic COVID-19 cases in South Korea using a probabilistic model. Int J Environ Res Public Health 2021;18:4946.
3. Yimer SA, Booij BB, Tobert G, Hebbeler A, Oloo P, Brangel P, et al. Rapid diagnostic test: a critical need for outbreak preparedness and response for high priority pathogens. BMJ Glob Health 2024;9:e014386.
4. Sung H, Yoo CK, Han MG, Lee SW, Lee H, Chun S, et al. Preparedness and rapid implementation of external quality assessment helped quickly increase COVID-19 testing capacity in the Republic of Korea. Clin Chem 2020;66:979–81.
5. Kim YJ, Sung H, Ki CS, Hur M. COVID-19 testing in South Korea: current status and the need for faster diagnostics. Ann Lab Med 2020;40:349–50.
6. Olalekan A, Iwalokun B, Akinloye OM, Popoola O, Samuel TA, Akinloye O. COVID-19 rapid diagnostic test could contain transmission in low- and middle-income countries. Afr J Lab Med 2020;9:1255.
7. Huh HJ, Hong KH, Kim TS, Song SH, Roh KH, Lee H, et al. Surveillance of coronavirus disease 2019 (COVID-19) testing in clinical laboratories in Korea. Ann Lab Med 2021;41:225–9.
8. Zayed RA, Omran D, Zayed AA. COVID-19 clinical and laboratory diagnosis overview. J Egypt Public Health Assoc 2021;96:25.
9. Reidy N, Coetzee H, Roche C, Brazil E, O’Sullivan L, Brady D, et al. SARS-CoV-2 testing and patient waiting times in the emergency department. Ir Med J 2022;115:633.
10. Jarrett M, Garrick R, Gaeta A, Lombardi D, Mayo R, McNulty P, et al. Pandemic preparedness: COVID-19 lessons learned in New York’s hospitals. Jt Comm J Qual Patient Saf 2022;48:475–91.
11. Kaufer AM, Theis T, Lau KA, Gray JL, Rawlinson WD. Laboratory biosafety measures involving SARS-CoV-2 and the classification as a Risk Group 3 biological agent. Pathology 2020;52:790–5.
12. Naeem W, Zeb H, Rashid MI. Laboratory biosafety measures of SARS-CoV-2 at containment level 2 with particular reference to its more infective variants. Biosaf Health 2022;4:11–4.
13. Lee K. Laboratory diagnosis of COVID-19 in Korea. Ewha Med J 2021;44:1–10.
14. Hong KH, Kim GJ, Roh KH, Sung H, Lee J, Kim SY, et al. Update of guidelines for laboratory diagnosis of COVID-19 in Korea. Ann Lab Med 2022;42:391–7.
15. Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2020;8:CD013705.
16. Kim SY, Lee J, Sung H, Lee H, Han MG, Yoo CK, et al. Pooling upper respiratory specimens for rapid mass screening of COVID-19 by real-time RT-PCR. Emerg Infect Dis 2020;26:246972.
17. Centers for Disease Control and Prevention. Interim guidance for use of pooling procedures in SARS-CoV-2 diagnostic, screening, and surveillance testing. https://stacks.cdc.gov/view/ cdc/90937 [Online] (last visited on 28 February 2025).
18. European Centre for Disease Prevention and Control (ECDC). Methodology for estimating point prevalence of SARS-CoV-2 infection by pooled RT-PCR testing. https://www.ecdc. europa.eu/en/publications-data/methodology-estimating-point-prevalence-sars-cov-2infection-pooled-rt-pcr [Online] (last visited on 28 May 2020).
19. Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol 2020;129:104455.
20. Lee J, Kim SY, Huh HJ, Kim N, Sung H, Lee H, et al. Clinical performance of the Standard Q COVID-19 rapid antigen test and simulation of its real-world application in Korea. Ann Lab Med 2021;41:588–92.