Departments of 1Laboratory Medicine, 2Research and Analysis, 3Pulmonology Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
Correspondence to Young Ah Kim, E-mail: yakim@nhimc.or.kr
Ann Clin Microbiol 2025;28(3):12. https://doi.org/10.5145/ACM.2025.28.3.1
Received on 17 March 2025, Revised on 12 May 2025, Accepted on 15 May 2025, Published on 24 July 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Background: The coronavirus disease 2019 (COVID-19) pandemic has pushed back years of progress that essential tuberculosis (TB) medical services provided in reducing the burden of TB. This study evaluated the clinical impact of the COVID-19 pandemic on TB management based on treatment discontinuation and mortality rates.
Methods: Two time intervals were included in this study: before the spread of COVID-19 (2018–2019) and during the pandemic phase of COVID-19 (2020–2021). Newly diagnosed patients with pulmonary TB (42,930 before and 32,094 during COVID-19) were included using the national reimbursement data from the health insurance service in Korea. Treatment discontinuation was defined as the discontinuation of treatment for more than 2 weeks during the initial intensive care phase or for more than 2 months during the maintenance phase. Mortality rates were compared before the spread and during the pandemic phase of COVID-19 in all patients and subgroups with various comorbidities. The Kaplan–Meier survival curve for each group of study participants was derived, and overall statistical significance was confirmed using the log-rank test.
Results: The treatment discontinuation rate over the entire period showed a trend of decreasing the time factor (-0.0084), decreasing the level change (-0.0166), and increasing the Interaction term (0.0039). These tendencies were similar during the initial intensive care and maintenance treatment periods, suggesting that the decreasing trend over time was significant, but the level change and slope change before the spread and during the pandemic phase of COVID-19 were not in agreement. The total mortality rates increased more during the COVID-19 pandemic (14.6% increase after 1 year and 18.9% increase after 2 years, based on 2020) than before COVID-19 (12.3% increase after 1 year and 16.1% increase after 2 years, based on 2018). Regarding the impact of comorbidities on TB mortality, malnutrition and diabetes showed a strong impact on the mortality with relative risks greater than 2 both in 1-year and 2-year mortalities.
Conclusion: This study verified the clinical impact of the COVID-19 pandemic on TB management. Therefore, establishing appropriate TB management policies is urgently needed for the future COVID-19-like pandemic situation.
Coronavirus disease, COVID-19, Mortality, Pulmonary tuberculosis, Treatment
Tuberculosis (TB) is a leading cause of mortality and considered a critical disease in public health [1]. Unfortunately, the recent coronavirus disease 2019 (COVID-19) pandemic situation could have a significant impact on the management of TB [2,3]. The World Health Organization (WHO) is concerned that the COVID-19 pandemic has pushed back years of progress that essential TB medical services provided [3], and identifying the impact of the COVID-19 pandemic on TB management in Korea is urgently needed to restore previous capacity.
According to the annual (WHO) Global TB report [4], the number of people diagnosed with TB has decreased significantly, with 18% fewer diagnoses in 2020 than in 2019, showing a significant difference from the estimates. In addition, the TB mortality rate, which has been declining every year since 2005, returned to the 2017 levels after the COVID-19 pandemic. Consequently, TB has become the second leading cause of death from a single infection after the COVID-19 pandemic. Accordingly, the WHO declared that the COVID-19 pandemic has reversed the years of progress that essential TB medical services provided in reducing the burden of TB.
The trend of decreased TB diagnosis was similar in Korea. According to a report by the Korea Disease Control and Prevention Agency [5], the number of newly diagnosed patients with TB in Korea decreased by 7.8% per year on average after peaking at 29,557 in 2011, showing a decrease of 58.9% in 2022, resulting in the number of new patients in 2022 being 31.7 per 100,000 people. The decline in TB cases in South Korea during the COVID-19 pandemic may be an extension of the existing declining trend and may also be the impact of COVID-19.
This study evaluated the clinical impact of the COVID-19 pandemic on TB management based on treatment discontinuation and mortality rates. These data will help establish appropriate TB management policies for future COVID-19-like pandemics.
It is a retrospective observational cohort study utilizing comprehensive national reimbursement data. It was described according to the Strengthening the Reporting of Observational Studies in Epidemiology statement.
The national reimbursement data from the health insurance service in Korea were searched by the authors in 2025. Data on newly diagnosed patients with pulmonary TB before and during the COVID-19 pandemic were collected.
Two time intervals were included in this study: before the spread of COVID-19 (2018–2019, control group) and during the pandemic phase of COVID-19 (2020–2021, experimental group). Newly diagnosed patients with pulmonary TB (42,930 patients in the control group and 32,094 patients in the experimental group) were included using the national reimbursement data from the health insurance service in Korea. Patients with pulmonary TB were selected using the V000 code, a special code for calculating fees for patients with TB by medical institutions. Only newly diagnosed patients were included after excluding those who were diagnosed and treated for TB before 2018. Patients with extrapulmonary TB were excluded from the study.
Multidrug-resistant (MDR)-TB cases were defined by the prescription of secondary drugs, such as streptomycin, amikacin, moxifloxacin, levofloxacin, prothionamide, cyclosporine, para-aminosalicylate, linezolid, bedaquiline, and delamanid. The presence or absence of comorbidities was determined using the diagnostic codes for alcohol dependence (F10), diabetes (E10–E14), malnutrition (E4), and human immunodeficiency virus (HIV) infection (B20–B24).
Outcome variables were TB treatment discontinuation and mortality rate due to pulmonary tuberculosis.
Analysis of treatment discontinuation: Treatment discontinuation was defined as when treatment was stopped for more than 2 weeks during the initial intensive care phase or for more than 2 months during the maintenance phase. The initial intensive care phase was defined as before 2 months in general and 6 months in patients with MDR-TB from the start of treatment. The maintenance phase was after 2 months in general and 6 months in patients with MDR-TB.
Analysis of mortality rate: Mortality rates before and during the pandemic phase of COVID-19 in all patients and subgroups with various comorbidities.
There was no selection bias since all target pulmonary TB population data were collected.
Sample size estimation was not done since all target subjects were included.
Regression analysis was used to analyze treatment discontinuation. The regression equation is Yt = β0 + β1T + β2Xt + β3TXt + Et, in which β0 is the reference level at T = 0, β1 is the change in outcome according to the change per month (the reference time), β2 is the change in level according to the intervention, and β3 is the change in slope according to the intervention.
We estimated the 2-year survival probability using the Kaplan–Meier method. The survival distributions between the two groups were compared using a log-rank test.
All analysis methods were subjected to a two-sided test, and the statistical significance level was set at P < 0.05. For statistical analyses, SAS 9.4 (SAS Institute Inc.) was used.
A total of 75,024 patients newly diagnosed with pulmonary TB were included, with 42,930 in the control group (before COVID-19) and 32,094 in the experimental group (during the pandemic phase of COVID-19).
Analysis of treatment discontinuation is summarized in Table 1. The treatment discontinuation rate over the entire period showed a trend of decreasing time factor (-0.0084), decreasing level change (-0.0166), and increasing interaction term (0.0039). During the initial intensive care period, the treatment discontinuation rate showed a trend of decreasing time factor (-0.0071), increasing level change before and during the pandemic phase of COVID-19 (0.0075), and increasing interaction term (0.0015). During the maintenance treatment period, the treatment discontinuation rate showed a trend of decreasing time factor (-0.0076), increasing level change (0.0103) before and during the pandemic phase of COVID-19, and increasing interaction term (0.004). The decreasing trend over time was statistically significant (P < 0.0001, 0.0121, and 0.0001, respectively); however, the level and slope changes before and during the pandemic phase of COVID-19 were not.
Table 1. Analysis of the treatment discontinuation
| Factors | Entire period | Initial intensive care period | Maintenance treatment period | |||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Intercept | -0.5190 | -2.3590 | -1.5494 | |||
| Time factor (Trend over time) | -0.0084 | < 0.0001 | -0.0071 | 0.0121 | -0.0076 | 0.0001 |
| Before and during the pandemic phase of COVID-19 (Level change) | -0.0166 | 0.6695 | 0.0075 | 0.9063 | 0.0103 | 0.8142 |
| Interaction term (Slope change) | 0.0039 | 0.1481 | 0.0015 | 0.7413 | 0.004 | 0.1949 |
Bold forms: statistically significant.
Abbreviation: COVID-19, coronavirus disease 2019.
Comparing the mortality rate of patients with pulmonary TB before (2018–2019) and during the pandemic phase of COVID-19 (2020–2021), the mortality rate increased during the pandemic phase of COVID-19. The mortality rates were 12.30% after 1 year and 16.14% after 2 years as the base of 2018, increasing to 14.63% after 1 year and 18.90% after 2 years as the base of 2020.
The mortality rates of patients without alcohol dependence, with alcohol dependence, without diabetes, with diabetes, without malnutrition, with malnutrition, without HIV infection, with HIV infection after 1 year and after 2 years as of 2018 and 2020 were presented in Table 2.
Table 2. Comparison of mortality rates in patients with pulmonary tuberculosis before and during the pandemic phase of COVID-19
| Groups | Time period | Before COVID-19 (%) [Lower limit, Upper limit] | During COVID-19 (%) [Lower limit, Upper limit] | Log-rank P |
|---|---|---|---|---|
| Total (n = 75,024) | After 1 year | 12.30 [12.14–12.46] | 14.63 [14.43–14.83] | < 0.0001 |
| After 2 years | 16.14 [15.96–16.32] | 18.90 [18.67–19.13] | ||
| Without alcohol dependence (n = 71,767) | After 1 year | 12.19 [12.13–12.35] | 14.51 [14.31–14.71] | < 0.0001 |
| After 2 years | 15.88 [15.70–16.06] | 18.68 [18.45–18.91] | ||
| With alcohol dependence (n = 3,257) | After 1 year | 14.89 [14.06–15.72] | 17.31 [16.30–18.32] | < 0.0001 |
| After 2 years | 21.96 [21.00–22.92] | 23.50 [22.31–24.69] | ||
| Without diabetes (n = 38,041) | After 1 year | 7.74 [7.56–7.92] | 9.19 [8.96–9.42] | < 0.0001 |
| After 2 years | 10.18 [9.98–10.38] | 12.00 [11.73–12.27] | ||
| With diabetes (n = 36,983) | After 1 year | 16.41 [17.17–17.68] | 19.63 [19.32–19.94] | < 0.0001 |
| After 2 years | 22.81 [22.52–23.10] | 25.27 [24.92–25.62] | ||
| Without malnutrition (n = 71,991) | After 1 year | 11.72 [11.58–11.86] | 13.84 [13.67–14.01] | < 0.0001 |
| After 2 years | 15.35 [15.17–15.53] | 18.04 [17.81–18.27] | ||
| With malnutrition (n = 3,033) | After 1 year | 26.88 [25.79–27.97] | 29.96 [28.73–31.19] | < 0.0001 |
| After 2 years | 35.86 [34.56–37.17] | 38.00 [36.62–39.38] | ||
| Without HIV infection (n = 74,913) | After 1 year | 12.30 [12.14–12.46] | 14.63 [14.43–14.83] | 0.7871 |
| After 2 years | 16.14 [15.96–16.32] | 18.89 [18.69–19.10] | ||
| With HIV infection (n = 111) | After 1 year | 13.84 [9.12–18.57] | 15.22 [9.92–20.52] | 0.3566 |
| After 2 years | 18.46 [13.65–23.27] | 22.70 [16.34–29.06] |
Abbreviations: COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus.
Bold form: statistically significant
The impact of each comorbidity on tuberculosis mortality before and during the COVID-19 pandemic was assessed. Fig. 1 presented the 1-year mortality risk ratios, while Fig. 2 displayed the 2-year mortality risk ratios for each comorbidity across both time periods.
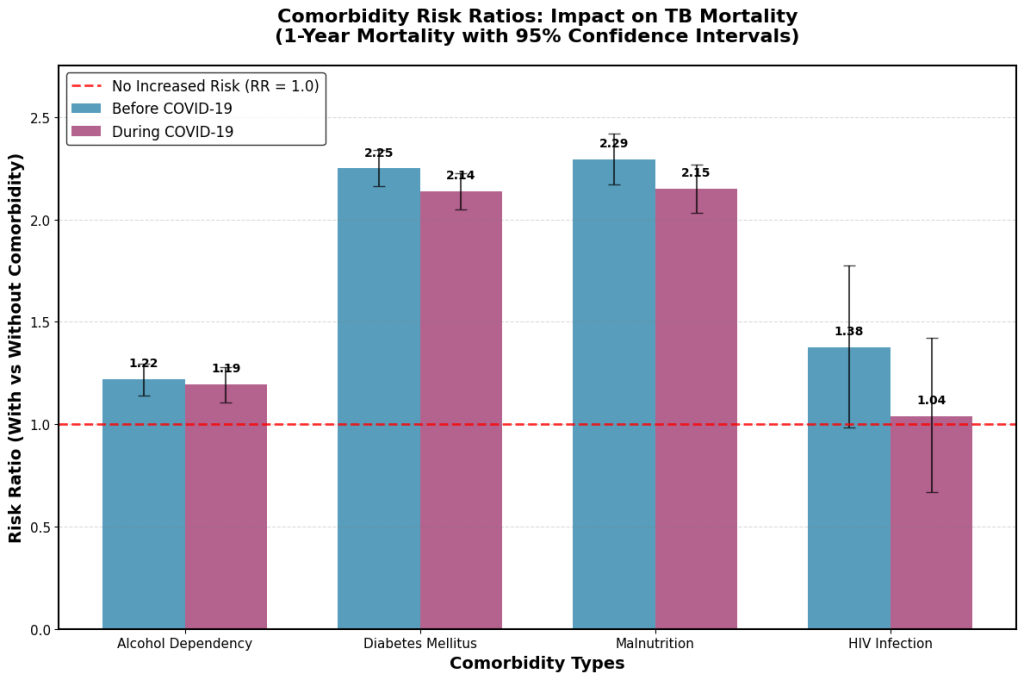
Fig. 1. Comorbidity-specific risk ratios for 1-year mortality in tuberculosis patients before and during the COVID-19 pandemic. TB, tuberculosis; COVID-19, coronavirus disease 2019.
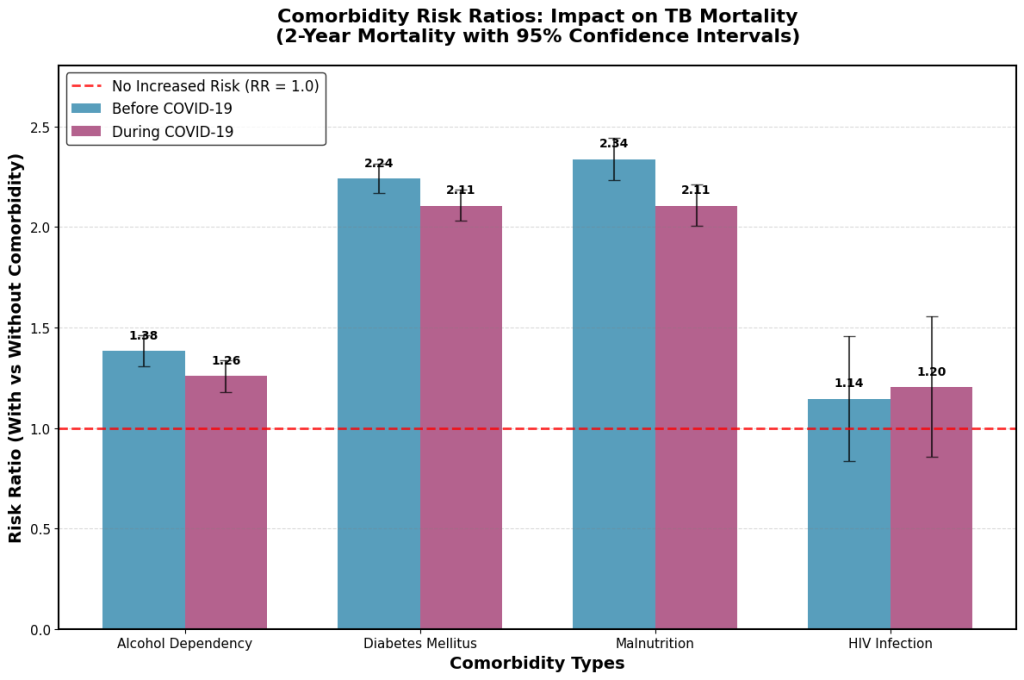
Fig. 2. Comorbidity-specific risk ratios for 2-year mortality in tuberculosis patients before and during the COVID-19 pandemic. TB, tuberculosis; COVID-19, coronavirus disease 2019.
Above findings suggest that the total mortality rates (after 1 year and after 2 years) increased more during the COVID-19 pandemic than before COVID-19 (P < 0.0001). Regardless of the presence or absence of alcohol dependence, diabetes, and malnutrition (except for HIV), the mortality rates increased more during the COVID-19 pandemic than before COVID-19 (Table 2).
Regarding the impact of comorbidities on TB mortality, malnutrition was associated with the highest oneyear mortality risk (risk ratio [RR]: 2.29 before COVID-19, decreasing slightly to 2.15 during the pandemic). Diabetes exhibited a similarly elevated risk (RR: 2.25 before COVID-19, decreasing to 2.14 during the pandemic). See Fig. 1. Malnutrition and diabetes showed strong impact also on the 2-year mortality (Fig. 2).
During the pandemic, the 1-year mortality rates were almost the same with or without HIV (RR: 1.04) but the 2-year mortality rates were not, suggesting the presence of temporary protective mechanisms during the early COVID-19 period. This finding highlights the need for ongoing monitoring of patients with HIV beyond the initial pandemic response, as well as further investigation into the factors responsible for the initial protection and its subsequent disappearance.
Fig. 1 may be used to inform short-term clinical planning and immediate response strategies during the COVID-19 pandemic. In contrast, Fig. 2 highlights the importance of long-term surveillance and sustained care protocols. Together, these findings illustrate the time-dependent effects of the COVID-19 pandemic on mortality risks in tuberculosis patients with various comorbidities.
Considering that there was no influenza epidemic and other respiratory viral infections decreased, transmission was reduced due to the wearing of masks, which could be effective in reducing the incidence of pulmonary TB. However, adverse effects (increased incidence) could be associated with several factors, such as lack of suspicion of pulmonary TB with similar symptoms, avoidance of tests due to the stigma effect of TB or COVID-19, refusal of COVID-19 isolation related to livelihood problems in low-income families, worsening nutritional status due to quarantine, increased family transmission during segregation, reduced medical care for non-emergency diseases, reduced accessibility to healthcare facilities, and limited resources (concentrated testing resources on COVID-19 management), which could delay the diagnosis and treatment of pulmonary TB during the COVID-19 pandemic.
Kwak et al. [6] reported a delay in TB notification following the COVID-19 pandemic. Although the infectious disease alert was raised to the highest level in response to the COVID-19 disease emergence, TB notifications during the first 18 weeks of 2020 decreased significantly from the same period each year from 2015 to 2019 [6]. A nationwide cross-sectional study using systematically collected data from the Korea Tuberculosis Cohort database in 2022 showed that healthcare delays in patients with TB increased during the first wave of the COVID-19 pandemic in Korea [7].
Maintaining TB management even during mass outbreaks of new infectious diseases, such as COVID-19 is essential. Many studies have focused on the TB management in the post-COVID-19 era [8–10]. A review of these studies suggests strengthening TB management in vulnerable groups, establishing management systems for patients with MDR-TB, providing community-based patient support, eliminating regional medical imbalances, setting up guidelines for TB management, expanding the foundation for TB research, applying digital technology, and integrating TB diagnostics into diagnostic and monitoring systems.
Korean Tuberculosis and Post-Tuberculosis Cohorts were constructed by linking the Korean National Tuberculosis Surveillance System and National Health Information Database, which are useful resources, but they can be used to analyze different measurement variables in an integrated manner depending on the data source [11]. We used Korean reimbursement data of the health insurance services, which included data from the majority of people with health insurance [12]. There are 1.3 trillion pieces of data on the National Health Insurance Corporation’s data-sharing service website, including qualifications and insurance for all citizens, details of hospital use, national health examination results, medical history, long-term care insurance for the elderly, status of nursing institutions, and registration information for cancer and rare and intractable diseases, which could be an excellent source for research after the characteristics of the data are understood [12].
Limitations may exist in data accuracy because such datasets reflect physician billing practices rather than the actual clinical status of patients. The mortality risk of HIV infection should be interpreted cautiously since the sample size (111) is smaller than other comorbidities.
This study verified the adverse clinical impacts of the COVID-19 pandemic on the management of TB. This study did not identify every factor associated with TB management. We believe that the treatment discontinuation rate and increased mortality during the COVID-19 pandemic could be indirect evidence suggestive of weakened TB management. The treatment discontinuation rate showed a significant decreasing trend over time, but the level change and slope change before and during the pandemic phase of COVID-19 were not in agreement. Mortality rates increased more during the pandemic phase of COVID-19, especially in patients with various comorbidities, although the HIV group in the pandemic phase of COVID-19 showed a greater increase in mortality than the HIV group before COVID-19 in the second year (not in the first year). This may be due to a decrease in the accessibility and use of medical resources for patients with TB symptoms during the pandemic. These data will help establish appropriate TB management policies for future COVID-19-like pandemics.
The Institutional Review Board of the National Health Insurance Service Ilsan Hospital approved this study (NHIMC 2023-10-022) and waived the requirement for informed consent.
No potential conflicts of interest relevant to this article were reported.
This study was funded by a grant from the National Health Insurance Service Ilsan Hospital (NHIMC2023-PR-006).
This study obtained permission to use the National Health Insurance Data Sharing Service (NHIMC-2023-10-022) and the datasets generated during the current study are available from the corresponding author upon request.
1. Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019;393:1642-56.

2. Aznar ML, Espinosa-Pereiro J, Saborit N, Jové N, Sánchez Martinez F, Pérez-Recio S, et al. Impact of the COVID-19 pandemic on tuberculosis management in Spain. Int J Infect Dis 2021;108:300-5.


3. Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: a rapid systematic review and meta-analysis. J Med Virol 2021;93:1946.


4. WHO. Global tuberculosis report 2021. https://www.who.int/publications/i/item/9789240037021 [Online] (last visited on 5 June 2025).
5. Lee HW, Kim JS, Park GJ, Choi HY. Report of tuberculosis patients in 2022. Public Health Wkly Rep 2023;16:931-49.
6. Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on tuberculosis notification, South Korea. Emerg Infect Dis 2020;26:2506-8.


7. Min J, Ko Y, Kim HW, Koo HK, Oh JY, Jeong YJ, et al. Increased healthcare delays in tuberculosis patients during the first wave of COVID-19 pandemic in Korea: a nationwide cross-sectional study. J Korean Med Sci 2022;37:e20.


8. Singh V and Chibale K. Strategies to combat multi-drug resistance in tuberculosis. Acc Chem Res 2021;54:2361-76.


9. Tale S and Meitei Soibam P. Care of tuberculosis patients in the times of COVID-19. Indian J Tuberc 2021;68:285-6.


10. Chapman HJ and Veras-Estévez BA. Lessons learned during the COVID-19 pandemic to strengthen TB infection control: a rapid review. Glob Health Sci Pract 2021;9:964-7.


11. Jeong D, Kang HY, Kim J, Lee H, Yoo BN, Kim HS, et al. Cohort profile: Korean Tuberculosis and Post-Tuberculosis Cohort constructed by linking the Korean National Tuberculosis Surveillance System and National Health Information Database. J Prev Med Public Health 2022;55:253-62.


12. Health Insurance Review & Assessment Service. HIRA Big Data Opening Portal. Health and medical big data. https://opendata.hira.or.kr/op/opb/selectHelhMedDataInfoView.do#none [Online] (last visited on 5 June 2025).
1. Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019;393:1642-56.

2. Aznar ML, Espinosa-Pereiro J, Saborit N, Jové N, Sánchez Martinez F, Pérez-Recio S, et al. Impact of the COVID-19 pandemic on tuberculosis management in Spain. Int J Infect Dis 2021;108:300-5.


3. Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: a rapid systematic review and meta-analysis. J Med Virol 2021;93:1946.


4. WHO. Global tuberculosis report 2021. https://www.who.int/publications/i/item/9789240037021 [Online] (last visited on 5 June 2025).
5. Lee HW, Kim JS, Park GJ, Choi HY. Report of tuberculosis patients in 2022. Public Health Wkly Rep 2023;16:931-49.
6. Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on tuberculosis notification, South Korea. Emerg Infect Dis 2020;26:2506-8.


7. Min J, Ko Y, Kim HW, Koo HK, Oh JY, Jeong YJ, et al. Increased healthcare delays in tuberculosis patients during the first wave of COVID-19 pandemic in Korea: a nationwide cross-sectional study. J Korean Med Sci 2022;37:e20.


8. Singh V and Chibale K. Strategies to combat multi-drug resistance in tuberculosis. Acc Chem Res 2021;54:2361-76.


9. Tale S and Meitei Soibam P. Care of tuberculosis patients in the times of COVID-19. Indian J Tuberc 2021;68:285-6.


10. Chapman HJ and Veras-Estévez BA. Lessons learned during the COVID-19 pandemic to strengthen TB infection control: a rapid review. Glob Health Sci Pract 2021;9:964-7.


11. Jeong D, Kang HY, Kim J, Lee H, Yoo BN, Kim HS, et al. Cohort profile: Korean Tuberculosis and Post-Tuberculosis Cohort constructed by linking the Korean National Tuberculosis Surveillance System and National Health Information Database. J Prev Med Public Health 2022;55:253-62.


12. Health Insurance Review & Assessment Service. HIRA Big Data Opening Portal. Health and medical big data. https://opendata.hira.or.kr/op/opb/selectHelhMedDataInfoView.do#none [Online] (last visited on 5 June 2025).