Sunjoo Kim1,2,3![]()
1Institute of Medical Science, Gyeongsang National University, Jinju, 2Department of Laboratory Medicine, Gyeongsang National University College of Medicine, Jinju, 3Department of Laboratory Medicine, Gyeongsang National University Changwon Hospital, Changwon, Korea
Corresponding to Sunjoo Kim, E-mail: sjkim8239@hanmail.net
Ann Clin Microbiol 2023;26(4):89-97. https://doi.org/10.5145/ACM.2023.26.4.89
Received on 29 November 2023, Revised on 15 December 2023, Accepted on 15 December 2023, Published on 20 December 2023.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Group A streptococci (GAS) cause diverse diseases ranging from mild to severe illnesses, and the global burden of GAS infections is enormous. Serological typing has been replaced by emm genotyping for the epidemiological study of GAS. Acute bacterial pharyngitis is a common illness, which requires either throat culture or rapid Ag test for diagnosis. Moreover, molecular point-of-care tests have been introduced owing to their higher sensitivity. Optimal diagnosis of bacterial pharyngitis is necessary for the adequate use of antibiotics. Although antimicrobial resistance (AMR) to erythromycin or clindamycin does not seem serious in Korea, it is very high in China, being reported at over 90%. Antibiotic surveillance and relevant education are necessary for primary clinical physicians and pediatricians. It is necessary to monitor AMR and develop a system for reporting the appearance of highly virulent diseases, such as necrotizing fasciitis or streptococcal toxic-shock syndrome, to the government authority.
Anti-bacterial agents, Bacterial drug resistance, Pharyngitis, Point-of-care testing, Streptococcus pyogenes
Group A streptococci (GAS, Streptococcus pyogenes) is an important pathogen for human beings as it leads to a range of diverse diseases, from mild diseases such as pharyngitis, scarlet fever, impetigo, and erysipelas, to severe illnesses such as necrotizing fasciitis, streptococcal toxic-shock syndrome (STSS), and puerperal sepsis. The most important sequelae of GAS infections are rheumatic fever (RF) and poststreptococcal glomerulonephritis (PSGN). Although these sequelae are rare in developed countries, they are still a burden in developing countries [1]. It has been estimated that more than 500,000 people have died of severe GAS infections [2]. As upper respiratory tract infections are the most common illness for human beings, optimal diagnosis and treatment of a sore throat is required. Over-usage of antibiotics for acute pharyngitis has been reported in many countries [3,4]. Considering the seriousness of antimicrobial resistance (AMR) worldwide, vigilant usage of antibiotics is necessary. In this study, the author reviewed the diseases, diagnosis, epidemiological methods, and AMR associated with GAS, especially from the perspective of the current situation in Korea.
Acute bacterial pharyngitis, mostly caused by GAS, is rather common in kindergarten- or young elementary school-age children [5,6]. However, it should be noted that most etiological agents for sore throats are viruses. Therefore, optimal diagnosis and treatment is crucial to avoid the misuse or overuse of antibiotics. It has been reported that more than 50% of sore throat patients visiting clinics were prescribed with antibiotics [3,4]. Considering that the proportion of GAS pharyngitis is less than 10% among sore throat patients, the overuse of antibiotics seems to be a serious problem. More education and consensus are needed for primary clinical physicians or pediatricians, in order to promote adequate diagnosis and treatment of this very common upper respiratory tract infection. With the introduction of a rapid Ag test (RAT) for streptococcal sore throat [7–9], the highly sensitive RAT can be used for the diagnosis of bacterial pharyngitis, in order to avoid ambiguous antibiotic treatment before receiving the bacterial culture result 1–2 days later.
Although scarlet fever is not a fatal illness, caregivers often worry about their children due to rashes spreading to the face or trunk. Pediatricians sometimes diagnose scarlet fever based on clinical manifestations if the throat culture is negative. There was a nationwide outbreak of scarlet fever in Korea in the mid-2010s [10,11], and emm4 or emm3 were the most common causes of scarlet fever [10,11]. As this is classified as a mandatory disease to report to the government agency, the statistics of scarlet fever are easily accessible.
Although RF and PSGN are classical GAS sequelae, at present they have almost disappeared in Korea; however, they may reappear at any time. For example, a resurgence of RF was reported in the USA during the 1980s, after lapsing for several decades [12]. Although these sequelae are a very serious public health concern in developing countries [1,2], we still do not understand their exact immunological mechanisms, as developing animal models for these sequelae is challenging [13].
GAS may cause necrotizing fasciitis (NF), which is rapidly progressing and fatal [14]. In NF, GAS spreads through the fascia rapidly. Early diagnosis and prompt surgical debridement are essential to cure necrotizing fasciitis.
STSS is the most fatal illness of GAS, with a mortality of over 40% [15], much higher than that of staphylococcal toxic-shock syndrome. STSS progresses rapidly throughout non-specific T-cell immune response [16]. Many different organs, such as the liver and kidney, may be damaged and become dysfunctional [16]. In particular, emm1 presented higher mortality compared to other emm types in Japan [15]. As this is the most fatal illness based on GAS, STSS as well as NF should be reported and monitored by the government authority, as is the case in Japan.
It is assumed that asymptomatic infections are widely present in elementary schools [17]. From an antiopacity factor (OF) analysis, more than 75% of school children harbored anti-OF in their sera [8]. The same percentage showed a higher than upper limit of normal (ULN; 200 IU/mL) anti-streptolysin O (ASO) level [18]. As these antibodies are proof of previous GAS infections, it can be assumed that a majority of school children have become immune to various M-types of GAS without evident symptoms [19]. Therefore, it is plausible that many carriers of GAS may undergo asymptomatic infections [17], or serve as a GAS reservoir [20] and build up herd immunity to the circulating M-types of GAS. There might be a tendency to more likely catch influenza in the case of these asymptomatic infections [21].
Bacterial culture is the gold standard for diagnosis of GAS infections or pharyngitis. However, it is largely affected by the sampling procedure, streaking technique on blood agar plate, and expertise of the technician [22]. Some GAS strains are not inhibited by a bacitracin disk [23], whereas non-GAS are sometimes inhibited by a bacitracin disk. Therefore, further confirmation tests using biochemical reaction, slide agglutination, or MALDI-TOF should be carried out in addition to bacitracin screening for optimal identification. In addition, for epidemiological survey in a given region, bacterial culture is needed to procure the GAS strains.
RAT has been used for testing sore throat patients in Western countries since the 1980s. RAT for streptococcal sore throat is the first developed point-of-care (POC) test for infectious diseases. Taking samples from the throat seems easy, simple, and RAT may prevent the decision to prescribe antibiotics without waiting 1–2 days for the throat culture results. Therefore, RAT for streptococcal sore throat has been widely adopted in primary clinics [3,5]. Due to the advancement of quality for in vitro diagnostic devices, the sensitivity of RAT is practically equivalent to that of bacterial culture at present [24,25]; however, the utilization of RAT for streptococcal sore throat is not well-documented in Korea, due to its low insurance cost and lack of education for physicians or pediatricians.
As molecular tests using PCR techniques give high sensitivity and short turn-around time [26], they are expected to become standard diagnostic tests for GAS pharyngitis [27]. Most importantly, any diagnostic test-such as culture, RAT, or molecular POC test-cannot differentiate between the carriers and true infections. Differentiation is not always necessary for symptomatic patients and the optimal diagnosis is largely affected by clinical expertise [5,28,29].
ASO is not commonly used at present, as very few cases of RF or PSGN are observed. For the diagnosis of acute pharyngitis, an ASO test is not recommended, as ASO will appear 2–3 weeks later. Furthermore, the physicians should be very careful when interpreting the ASO result, as the ULN of ASO differs by age group [30]. The ULN of ASO is defined as 80% of the cumulative ASO in the population, and has been found to be over 400 IU/mL for elementary school children [18,19].
Although T- and M-typing have previously been used for the serological epidemiological study of GAS in the past, these tests became disused after the introduction of emm genotyping. T-typing is based on slide agglutination by using commercially available anti-T sera (Fig. 1). The T-protein is not associated with disease and presents a cross-reaction [7]. M-typing is based on double immuno-diffusion on agarose gel using anti-M sera produced from rabbits (Fig. 1). The M-protein is related to disease and is the most important virulence factor of GAS [7]. However, some strains do not produce anti-M sera in rabbits; instead, they make horse sera opaque. This protein is called OF [7]. Anti-OF sera can be procured from human beings who have been infected with a specific OF type [8].

(a) Slide agglutination for T-typing. Extracted T Ag (red broth) is reacted on the slide with the commecially available anti-T sera.
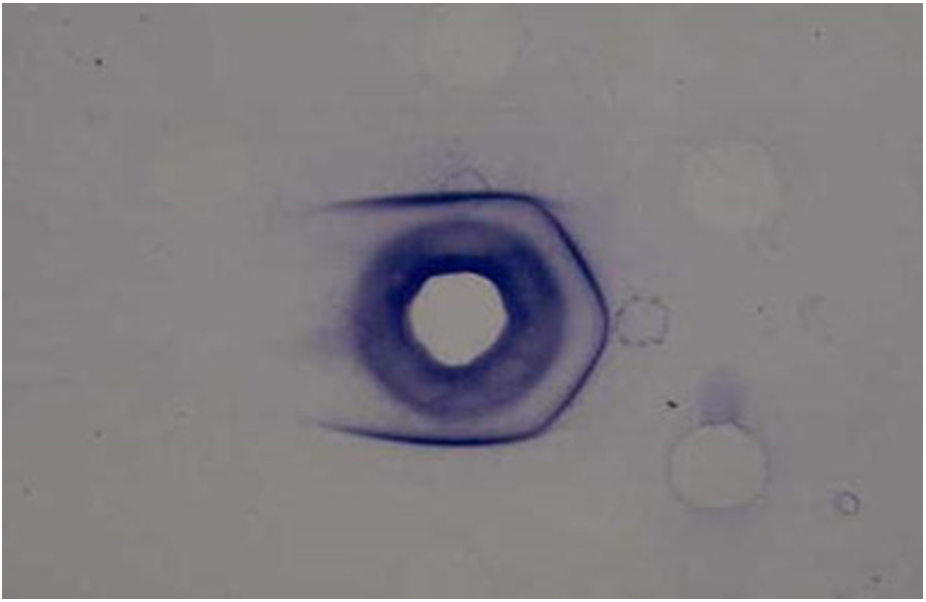
(b) Double immunodiffusion for M typing. Extracted M proteins diffuse in the agarose gel and make precipitation with anti-M sera in the center. Four out of 6 show positive.
Fig. 1. Serotyping of group A streptococci by (a) T typing and (b) M typing.
The serotyping procedure is time-consuming and is difficult to carry out in the clinical laboratory. Serotyping was replaced by emm genotyping in early 2000s. Emm is the M protein-encoding gene, which has hypervariable sequences at the 5′-N-terminal with a size of 500–1500 bp [9]. More than 220 emm genotypes have been reported [31]. Emm genotyping is rather simple and easy to perform for the molecular epidemiological study of GAS. Although GAS infections are type-specific [7], indicating that an individual may not be protected against another M-type, but only immune to the same M-type, several emm genotypes have presented cross-immunity characteristics. These emm genotypes are classified as the emm clades [32], and are important for the development of vaccines. At present, a 30-valent vaccine is under development [10,11,32]. Dynamic changes in distribution of emm genotypes have been observed in regions or countries, suggesting the waxing and waning of circulating GAS M-types in the community [33,34].
With the technical advancement and lower cost, WGS is becoming more available. Through WGS, more information regarding not only emm genotypes, but also sequence types, AMR genes, and virulence genes can be obtained. In addition, through WGS, we can understand the size of the whole genome, as well as similarities and differences between genomes [9,20]. With the widespread application of WGS technology in routine clinical laboratories, this technique can provide multi-faceted information on GAS.
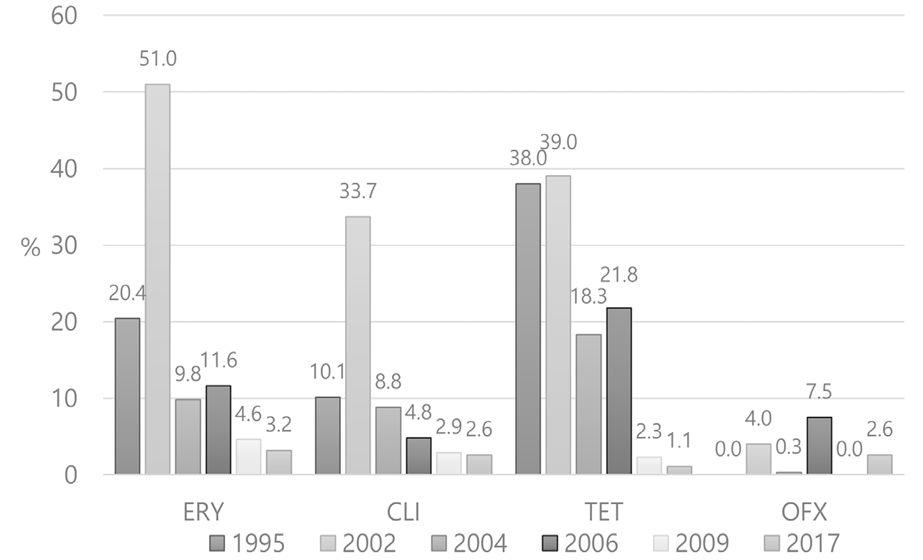
Fortunately, AMR to erythromycin and clindamycin is not yet a serious concern in Korea. AMR to these antibiotics is reported in less than 5%, which has remained stable since 2004 [6,35] (Fig. 2). As antibiotic-resistant strains such as emm12 have decreased and antibiotic-susceptible strains, such as emm4, are prevalent these days, the change in distribution of emm genotypes may have affected the reported decrease in AMR [32,36,37]. As many new macrolides, such as azithromycin and clarithromycin, have widely been used for respiratory tract infections in the clinics, more efforts for keen use of these drugs are needed. Furthermore, AMR to erythromycin and clindamycin has been reported to be around 25% in the United States [36] and as high as 95% in China [36,38,39]. This is a serious concern, considering that these drugs should be secured for patients who are allergic to penicillin. Still, no penicillin-resistant GAS has been reported to date [36,38,39]. Although penicillin or amoxicillin are regarded as drugs of choice for bacterial pharyngitis [5,40], there are cases of treatment failure when GAS is located inside the cells, as these drugs are less effective in such a scenario [41].
GAS is an important pathogen causing diverse diseases. Although GAS is the most common cause of bacterial pharyngitis, the prescription of antibiotics for sore throats seems to be much higher than necessary. Antibiotic stewardship and education regarding optimal diagnostic tests are required for primary physicians or pediatricians. RAT seems more practical than throat culture, the results of which can be returned in about 2 days. Molecular POC testing is expected to become more popular in clinics and hospitals due to its feasibility and high sensitivity. STSS or NF should be reported to the appropriate government authority in order to monitor the spread of highly virulent strains in the community. At present, AMR does not seem to be a serious concern in Korea. However, AMR may increase if we do not pay attention to the right usage of antibiotics and/or if resistant emm genotypes re-appear.
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
This research was supported by the National Research Foundation (NRF) of Korea (NRF2021R1I1A3044483 and NRF-2021M3E5E3080382). The funders had no role in the study design, data collection, interpretation, or decision to submit the manuscript for publication.
1. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017;377;713-22. 

2. Lv M, Jiang S, Liao D, Lin Z, Chen H, Zhang J. Global burden of rheumatic heart disease and its association with socioeconomic development status, 1990–2019. Eur J Prev Cardiol 2022;29:1425-34. 

3. Luo R, Sickler J, Vahidnia F, Lee YC, Frogner B, Thompson M. Diagnosis and management of group A streptococcal pharyngitis in the United States, 2011–2015. BMC Infect Dis 2019; 19:1-9. 


4. Kim J, Park J, Kim BY, Kim DS. The trend of acute respiratory tract infections and antibiotic prescription rates in outpatient settings using health insurance data. Korean J Physiol Pharmacol 2017;27:186-94. 
5. Kalra MG, Higgins KE, Perez ED. Common questions about streptococcal pharyngitis. Am Fam Physician 2016;94:24-31.
6. Kim S, Lee S, Park H, Kim S. Predominance of emm4 and antibiotic resistance of Streptococcus pyogenes in acute pharyngitis in a southern region of Korea. Int J Med Microbiol 2019;68:1053-8. 

7. Johnson DR, Kaplan EL, VanGheem A, Facklam RR, Beall B. Characterization of group A streptococci (Streptococcus pyogenes): correlation of M-protein and emm-gene type with T-protein agglutination pattern and serum opacity factor. J Med Microbiol 2006;55:157-64. 

8. Kim S and Lee NY. Epidemiological usefulness of anti-opacity factor antibody screening in schoolchildren. J Clin Microbiol 2001;39:1316-8. 


9. Brouwer S, Rivera-Hernandez T, Curren BF, Harbison-Price N, De Oliveira DM, Jespersen MG, et al. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat Rev Microbiol 2023;21:431-47. 



10. Pastural É, McNeil SA, MacKinnon-Cameron D, Ye L, Langley JM, Stewart R, et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: a randomized, controlled phase I study. Vaccine 2020;38:1384-92. 

11. Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 2011;29:8175-8. 


12. Hosier DM, Craenen JM, Teske DW, Wheller JJ. Resurgence of acute rheumatic fever. Am J Dis Child 1987;141:730-3. 

13. Rush CM, Govan BL, Sikder S, Williams NL, Ketheesan N. Animal models to investigate the pathogenesis of rheumatic heart disease. Front Pediatr 2014;2:116. 


14. Bruun T, Kittang B, De Hoog B, Aardal S, Flaatten H, Langeland N, et al. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect 2013;19:E545-50. 

15. Ikebe T, Tominaga K, Shima T, Okuno R, Kubota H, Ogata K, et al. Increased prevalence of group A streptococcus isolates in streptococcal toxic shock syndrome cases in Japan from 2010 to 2012. Epidemiol Infect 2015;143:864-72. 


16. Schmitz M, Roux X, Huttner B, Pugin J. Streptococcal toxic shock syndrome in the intensive care unit. Ann Intensive Care 2018;8:1-10. 


17. Cordery R, Purba AK, Begum L, Mills E, Mosavie M, Vieira A, et al. Frequency of transmission, asymptomatic shedding, and airborne spread of Streptococcus pyogenes in schoolchildren exposed to scarlet fever: a prospective, longitudinal, multicohort, molecular epidemiological, contact-tracing study in England, UK. Lancet Microbe 2022;3:e366-75. 

18. Kim SJ. Distribution and upper limit of normal antistreptolysin O concentrations of school. Korean J Infect Dis 1997;233-8.
19. Bennett J, Moreland NJ, Williamson DA, Carapetis J, Crane J, Whitcombe AL, et al. Comparison of group A streptococcal titres in healthy children and those with pharyngitis and skin infections. J Infect 2022;84:24-30. 

20. Lacey JA, Marcato AJ, Chisholm RH, Campbell PT, Zachreson C, Price DJ, et al. Evaluating the role of asymptomatic throat carriage of Streptococcus pyogenes in impetigo transmission in remote Aboriginal communities in Northern Territory, Australia: a retrospective genomic analysis. Lancet Microbe 2023;4:E524-33. 

21. Keeley AJ, Groves D, Armitage EP, Senghore E, Jagne YJ, Sallah HJ, et al. Streptococcus pyogenes colonization in children aged 24–59 months in the Gambia: impact of live attenuated influenza vaccine and associated serological responses. J Infect Dis 2023;228:957-65. 


22. Kim SJ. Optimal site of throat swab for the isolation of beta-hemolytic streptococci. J Korean Med Sci 1993;8:453-7. 


23. Pires R, Rolo D, Mato R, Feio de Almeida J, Johansson C, Henriques-Normark B, et al. Resistance to bacitracin in Streptococcus pyogenes from oropharyngeal colonization and noninvasive infections in Portugal was caused by two clones of distinct virulence genotypes. FEMS Microbiol Lett 2009;296:235-40. 

24. Berry GJ, Miller CR, Prats MM, Marquez C, Oladipo OO, Loeffelholz MJ, et al. Comparison of the Alere i Strep A test and the BD Veritor system in the detection of group A Streptococcus and the hypothetical impact of results on antibiotic utilization. J Clin Microbiol 2018;56:e01310-7. 


25. Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst Rev 2016;7:CD010502. 
26. Close RM, Sutcliffe CG, Galdun P, Reid A, Askew MR, Davidson AM, et al. Point-of-care molecular diagnostics for the detection of group A Streptococcus in non-invasive skin and soft tissue infections: a validation study. Diagn Microbiol Infect Dis 2022;103:115729. 

27. May L, Sickler J, Robbins EM, Tang S, Chugh K, Tran N. The impact of point-of-care polymerase chain reaction testing on prescribing practices in primary care for management of Strep A: a retrospective before–after study. Open Forum Infect Dis 2022;9:ofac147. 


28. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. Does this patient have strep throat? JAMA 2000;284:2912-8. 

29. Jo SA, Ma SH, Kim S. Diagnostic impact of clinical manifestations of group A Streptococcal pharyngitis. Infect Chemother 2021;53:553. 


30. Kim SJ, Chung MA, Chung HJ, Kim YJ, Maeng KY. Distribution and upper limit of normal antistreptolysin O concentrations according to age. Korean J Infect Dis 1998;392-6.
31. McMillan DJ, Drèze PA, Vu T, Bessen DE, Guglielmini J, Steer AC, et al. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin Microbiol Infect 2013;19:E222-9. 


32. Rafei R, Iaali RA, Osman M, Dabboussi F, Hamze M. A global snapshot on the prevalent macrolide-resistant emm types of Group A Streptococcus worldwide, their phenotypes and their resistance marker genotypes during the last two decades: a systematic review. Infect Genet Evol 2022;99:105258. 

33. Choi JH, Yang NR, Lee WJ, Lee H, Choi EH, Lee HJ. Distribution of emm types among group A Streptococcus isolates from children in Korea. Diagn Microbiol Infect Dis 2015;82:26-31. 

34. Kim HN, Kim J, Jang WS, Nam J, Lim CS. Performance evaluation of three rapid antigen tests for the diagnosis of group A Streptococci. BMJ Open 2019;9:e025438. 


35. Cho YN, Park SE, Cho EY, Cho HK, Park JY, Kang HM, et al. Distribution of emm genotypes in group A streptococcus isolates of Korean children from 2012 to 2019. J Microbiol Immunol Infect 2022;55:671-7. 

36. Li Y, Rivers J, Mathis S, Li Z, McGee L, Chochua S, et al. Continued increase of erythromycin nonsusceptibility and clindamycin nonsusceptibility among invasive group A streptococci driven by genomic clusters, United States, 2018–2019. Clin Infect Dis 2023;76:e1266-9. 
37. Wajima T, Morozumi M, Chiba N, Shouji M, Iwata S, Sakata H, et al. Associations of macrolide and fluoroquinolone resistance with molecular typing in Streptococcus pyogenes from invasive infections, 2010–2012. Int J Antimicrob Agents 2013;42:447-9. 

38. Lu B, Fang Y, Fan Y, Chen X, Wang J, Zeng J, et al. High prevalence of macrolide-resistance and molecular characterization of Streptococcus pyogenes isolates circulating in China from 2009 to 2016. Front Microbiol 2017;8:1052. 


39. Li H, Zhou L, Zhao Y, Ma L, Liu X, Hu J. Molecular epidemiology and antimicrobial resistance of group a streptococcus recovered from patients in Beijing, China. BMC Infect Dis 2020;20:1-9. 


40. Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55:e86-102. 


41. McGuire E, Li A, Collin SM, Decraene V, Cook M, Padfield S, et al. Time to negative throat culture following initiation of antibiotics for pharyngeal group A Streptococcus: a systematic review and meta-analysis up to October 2021 to inform public health control measures. Euro Surveill 2023;28:2200573. 


1. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017;377;713-22.
2. Lv M, Jiang S, Liao D, Lin Z, Chen H, Zhang J. Global burden of rheumatic heart disease and its association with socioeconomic development status, 1990–2019. Eur J Prev Cardiol 2022;29:1425-34.
3. Luo R, Sickler J, Vahidnia F, Lee YC, Frogner B, Thompson M. Diagnosis and management of group A streptococcal pharyngitis in the United States, 2011–2015. BMC Infect Dis 2019; 19:1-9.
4. Kim J, Park J, Kim BY, Kim DS. The trend of acute respiratory tract infections and antibiotic prescription rates in outpatient settings using health insurance data. Korean J Physiol Pharmacol 2017;27:186-94.
5. Kalra MG, Higgins KE, Perez ED. Common questions about streptococcal pharyngitis. Am Fam Physician 2016;94:24-31.
6. Kim S, Lee S, Park H, Kim S. Predominance of emm4 and antibiotic resistance of Streptococcus pyogenes in acute pharyngitis in a southern region of Korea. Int J Med Microbiol 2019;68:1053-8.
7. Johnson DR, Kaplan EL, VanGheem A, Facklam RR, Beall B. Characterization of group A streptococci (Streptococcus pyogenes): correlation of M-protein and emm-gene type with T-protein agglutination pattern and serum opacity factor. J Med Microbiol 2006;55:157-64.
8. Kim S and Lee NY. Epidemiological usefulness of anti-opacity factor antibody screening in schoolchildren. J Clin Microbiol 2001;39:1316-8.
9. Brouwer S, Rivera-Hernandez T, Curren BF, Harbison-Price N, De Oliveira DM, Jespersen MG, et al. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat Rev Microbiol 2023;21:431-47.
10. Pastural É, McNeil SA, MacKinnon-Cameron D, Ye L, Langley JM, Stewart R, et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: a randomized, controlled phase I study. Vaccine 2020;38:1384-92.
11. Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 2011;29:8175-8.
12. Hosier DM, Craenen JM, Teske DW, Wheller JJ. Resurgence of acute rheumatic fever. Am J Dis Child 1987;141:730-3.
13. Rush CM, Govan BL, Sikder S, Williams NL, Ketheesan N. Animal models to investigate the pathogenesis of rheumatic heart disease. Front Pediatr 2014;2:116.
14. Bruun T, Kittang B, De Hoog B, Aardal S, Flaatten H, Langeland N, et al. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect 2013;19:E545-50.
15. Ikebe T, Tominaga K, Shima T, Okuno R, Kubota H, Ogata K, et al. Increased prevalence of group A streptococcus isolates in streptococcal toxic shock syndrome cases in Japan from 2010 to 2012. Epidemiol Infect 2015;143:864-72.
16. Schmitz M, Roux X, Huttner B, Pugin J. Streptococcal toxic shock syndrome in the intensive care unit. Ann Intensive Care 2018;8:1-10.
17. Cordery R, Purba AK, Begum L, Mills E, Mosavie M, Vieira A, et al. Frequency of transmission, asymptomatic shedding, and airborne spread of Streptococcus pyogenes in schoolchildren exposed to scarlet fever: a prospective, longitudinal, multicohort, molecular epidemiological, contact-tracing study in England, UK. Lancet Microbe 2022;3:e366-75.
18. Kim SJ. Distribution and upper limit of normal antistreptolysin O concentrations of school. Korean J Infect Dis 1997;233-8.
19. Bennett J, Moreland NJ, Williamson DA, Carapetis J, Crane J, Whitcombe AL, et al. Comparison of group A streptococcal titres in healthy children and those with pharyngitis and skin infections. J Infect 2022;84:24-30.
20. Lacey JA, Marcato AJ, Chisholm RH, Campbell PT, Zachreson C, Price DJ, et al. Evaluating the role of asymptomatic throat carriage of Streptococcus pyogenes in impetigo transmission in remote Aboriginal communities in Northern Territory, Australia: a retrospective genomic analysis. Lancet Microbe 2023;4:E524-33.
21. Keeley AJ, Groves D, Armitage EP, Senghore E, Jagne YJ, Sallah HJ, et al. Streptococcus pyogenes colonization in children aged 24–59 months in the Gambia: impact of live attenuated influenza vaccine and associated serological responses. J Infect Dis 2023;228:957-65.
22. Kim SJ. Optimal site of throat swab for the isolation of beta-hemolytic streptococci. J Korean Med Sci 1993;8:453-7.
23. Pires R, Rolo D, Mato R, Feio de Almeida J, Johansson C, Henriques-Normark B, et al. Resistance to bacitracin in Streptococcus pyogenes from oropharyngeal colonization and noninvasive infections in Portugal was caused by two clones of distinct virulence genotypes. FEMS Microbiol Lett 2009;296:235-40.
24. Berry GJ, Miller CR, Prats MM, Marquez C, Oladipo OO, Loeffelholz MJ, et al. Comparison of the Alere i Strep A test and the BD Veritor system in the detection of group A Streptococcus and the hypothetical impact of results on antibiotic utilization. J Clin Microbiol 2018;56:e01310-7.
25. Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst Rev 2016;7:CD010502.
26. Close RM, Sutcliffe CG, Galdun P, Reid A, Askew MR, Davidson AM, et al. Point-of-care molecular diagnostics for the detection of group A Streptococcus in non-invasive skin and soft tissue infections: a validation study. Diagn Microbiol Infect Dis 2022;103:115729.
27. May L, Sickler J, Robbins EM, Tang S, Chugh K, Tran N. The impact of point-of-care polymerase chain reaction testing on prescribing practices in primary care for management of Strep A: a retrospective before–after study. Open Forum Infect Dis 2022;9:ofac147.
28. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. Does this patient have strep throat? JAMA 2000;284:2912-8.
29. Jo SA, Ma SH, Kim S. Diagnostic impact of clinical manifestations of group A Streptococcal pharyngitis. Infect Chemother 2021;53:553.
30. Kim SJ, Chung MA, Chung HJ, Kim YJ, Maeng KY. Distribution and upper limit of normal antistreptolysin O concentrations according to age. Korean J Infect Dis 1998;392-6.
31. McMillan DJ, Drèze PA, Vu T, Bessen DE, Guglielmini J, Steer AC, et al. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin Microbiol Infect 2013;19:E222-9.
32. Rafei R, Iaali RA, Osman M, Dabboussi F, Hamze M. A global snapshot on the prevalent macrolide-resistant emm types of Group A Streptococcus worldwide, their phenotypes and their resistance marker genotypes during the last two decades: a systematic review. Infect Genet Evol 2022;99:105258.
33. Choi JH, Yang NR, Lee WJ, Lee H, Choi EH, Lee HJ. Distribution of emm types among group A Streptococcus isolates from children in Korea. Diagn Microbiol Infect Dis 2015;82:26-31.
34. Kim HN, Kim J, Jang WS, Nam J, Lim CS. Performance evaluation of three rapid antigen tests for the diagnosis of group A Streptococci. BMJ Open 2019;9:e025438.
35. Cho YN, Park SE, Cho EY, Cho HK, Park JY, Kang HM, et al. Distribution of emm genotypes in group A streptococcus isolates of Korean children from 2012 to 2019. J Microbiol Immunol Infect 2022;55:671-7.
36. Li Y, Rivers J, Mathis S, Li Z, McGee L, Chochua S, et al. Continued increase of erythromycin nonsusceptibility and clindamycin nonsusceptibility among invasive group A streptococci driven by genomic clusters, United States, 2018–2019. Clin Infect Dis 2023;76:e1266-9.
37. Wajima T, Morozumi M, Chiba N, Shouji M, Iwata S, Sakata H, et al. Associations of macrolide and fluoroquinolone resistance with molecular typing in Streptococcus pyogenes from invasive infections, 2010–2012. Int J Antimicrob Agents 2013;42:447-9.
38. Lu B, Fang Y, Fan Y, Chen X, Wang J, Zeng J, et al. High prevalence of macrolide-resistance and molecular characterization of Streptococcus pyogenes isolates circulating in China from 2009 to 2016. Front Microbiol 2017;8:1052.
39. Li H, Zhou L, Zhao Y, Ma L, Liu X, Hu J. Molecular epidemiology and antimicrobial resistance of group a streptococcus recovered from patients in Beijing, China. BMC Infect Dis 2020;20:1-9.
40. Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55:e86-102.
41. McGuire E, Li A, Collin SM, Decraene V, Cook M, Padfield S, et al. Time to negative throat culture following initiation of antibiotics for pharyngeal group A Streptococcus: a systematic review and meta-analysis up to October 2021 to inform public health control measures. Euro Surveill 2023;28:2200573.