1Department of Laboratory Medicine, Kyung Hee University Hospital, Seoul, Korea
2Department of Laboratory Medicine, Kyung Hee University College of Medicine, Kyung Hee University Hospital, Seoul, Korea
Correspondence to Young Jin Kim, E-mail: khmclab@gmail.com
Ann Clin Microbiol 2025;28(3):17. https://doi.org/10.5145/ACM.2025.28.3.6
Received on 7 July 2025, Revised on 4 September 2025, Accepted on 4 September 2025, Published on 20 September 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Latent tuberculosis (TB) refers to a state in which an individual is infected with Mycobacterium tuberculosis but shows no clinical symptoms. The World Health Organization estimates that 23% of the global population has latent TB, which poses a significant public health challenge owing to the risk of progression to active TB. Diagnosis of latent TB involves tests, such as the tuberculin skin test (TST) and interferon-gamma release assays (IGRAs). The TST can yield false positives due to prior Bacillus Calmette-Guérin (BCG) vaccination, whereas IGRAs offer higher specificity and are unaffected by BCG vaccination. Factors, such as age and recent vaccinations, can affect test performance. Treatment with isoniazid and rifampicin is recommended for those diagnosed, as it has been shown to prevent 80%-90% of active TB cases, although more extended follow-up studies are needed to confirm its long-term efficacy. Indeterminate IGRA results, especially in immunocompromised individuals, add complexity to the diagnosis and treatment decisions, highlighting the need for careful interpretation. Further research is vital to improve the diagnostic accuracy, interpretation, and treatment effectiveness.
Interferon-gamma release assay, Immunocompromised host, Latent tuberculosis, Tuberculin test
Latent tuberculosis (TB) refers to a state in which an individual is infected with Mycobacterium tuberculosis and has a sustained immune response, yet shows no clinical symptoms or signs of the disease. According to the World Health Organization (WHO), it is defined as the presence of evidence of M. tuberculosis infection without any clinical, radiological, or microbiological evidence of active TB disease [1].
After M. tuberculosis enters the body, it undergoes several stages before progressing to active TB. To describe the continuous disease progression that can move bidirectionally between TB infection and active TB, the concepts of ‘incipient TB’ and ‘subclinical TB’ have been introduced [2]. Incipient TB refers to a state in which the immune system suppresses M. tuberculosis, yet the bacteria proliferate within granulomas, posing a potential risk of future progression to active TB. At this stage, M. tuberculosis is not yet expelled; therefore, there is no transmission risk and there are no radiological signs, making it undiagnosable through clinical tests. Subclinical TB is characterized by the beginning of M. tuberculosis expulsion from granulomas, where sensitive diagnostic tests may detect M. tuberculosis and radiological traces may be observed. However, there are no clinical symptoms, and sputum is typically not produced, making it challenging to obtain proper clinical samples for diagnosis. Latent TB tests yield positive results throughout the TB infection continuum, from TB infection to incipient TB, subclinical TB, and active TB. However, these stages cannot be distinguished. The infectivity and serological test results are shown schematically in Fig. 1. Nonetheless, they are used to screen patients exposed to M. tuberculosis, even in the absence of microbiological or radiological evidence [3].
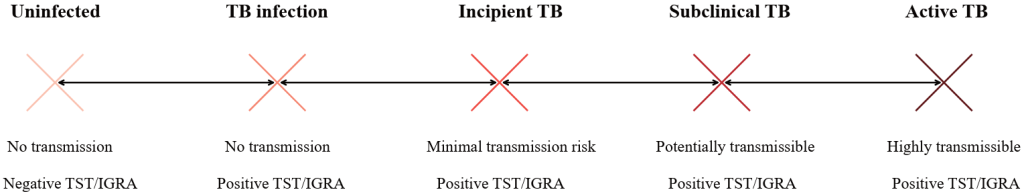
Fig. 1. Schematic representation of the tuberculosis infection continuum in relation to infectivity and immunologic test results. Abbreviations: TB, tuberculosis; TST/IGRA, tuberculin skin test/interferon-gamma release assays
This narrative review aims to describe the principles and clinical applications of interferon-gamma release assays (IGRAs) in latent tuberculosis diagnosis1. It examines their advantages over tuberculin skin test (TST), factors affecting performance, and the clinical significance of indeterminate results, emphasizing the need for improved diagnostic accuracy and treatment effectiveness.
Without a gold standard for diagnosing latent TB, accurately assessing the global burden is challenging. However, a 2016 WHO report estimated that 23% of the global population was affected based on TST results [1]. A meta-analysis of studies published between 2005 and 2018 reported an infection rate of 24.8% (19.7%-30.0%) based on IGRAs and 21.2% (17.9%-24.4%) with TST [3]. In South Korea, according to a 2022 IGRA report by the Korea Disease Control and Prevention Agency, 20.0% of individuals tested positive for latent TB in group facilities. However, the positive rates varied among different groups; the highest positive rate was observed in correctional facilities at 37.1%, while military units had a rate of 9.6%, and schools had a rate of only 6.7% [4]. In another study conducted in 2019 using IGRA, the positivity rate among healthcare workers was 9.8%, and there was no significant association between working in TB-related departments and positive latent TB infection [5].
The burden of active TB is higher in the low- and middle-income countries [6]. According to WHO reports, although the incidence of active TB has decreased, the number of individuals with latent TB infection remains alarmingly high, particularly among vulnerable groups, such as those with human immunodeficiency virus (HIV) infection and healthcare workers [7]. Although patients with latent TB should be monitored for progression to active TB, not all individuals with latent TB progress to active TB [8]. The annual incidence of active TB among untreated individuals with latent infection and positive IGRA results varies widely depending on the study population. Pooled data from TB contacts reported an incidence of 17.0 per 1000 person-years [9]. In a rural Chinese cohort with QFT positivity, the incidence was 8.7 per 1000 person-years [10]. A U.S. cohort of high-risk immigrants demonstrated a lower rate of 1.4 per 1000 person-years [11]. Among HIV-infected individuals with positive IGRA results, the incidence reached 15.2 per 1000 person-years [12]. Groups with a higher rate of progression to active TB include those with HIV/AIDS, those undergoing immunosuppressive therapy, and individuals with a history of active TB [7].
According to the results of a national latent TB screening program conducted on one million people in South Korea in 2017 and 2018 and followed up for an average of 2.2 years, the incidence of active TB based on IGRA results was reported as 10.6 per 100,000 person-years for IGRA-negative individuals. However, it was significantly higher at 173.1 per 100,000 person-years in those who were IGRA-positive. Nonetheless, for IGRA-positive individuals who receive appropriate treatment, the incidence could be reduced to 31.8 per 100,000 person-years [13]. Therefore, active screening and treatment programs should be implemented.
Tests for latent TB include the TST and blood-based IGRAs. The TST involves injecting a purified protein derivative (PPD, a soluble growth product extracted from TB bacteria), intradermally and measuring the diameter of induration 48-72 hours later [14]. A diameter of 10 mm or more is considered positive [15]. False positives can occur due to atypical mycobacteria or a history of Bacillus Calmette-Guérin (BCG) vaccination. False negatives can be caused by viral infections (e.g., measles, mumps, chickenpox, and influenza), immunosuppression (due to medication or protein malnutrition), aging, stress, coexisting conditions (e.g., sarcoidosis, malignant tumors, and lymphoma), HIV infection, live virus vaccinations, technical errors, or reagent degradation [15].
IGRA is a method for diagnosing TB by measuring interferon gamma released from T lymphocytes sensitized to M. tuberculosis antigens [1,16]. Its advantages include higher sensitivity compared to the TST, applicability in immunocompromised individuals, unaffected by the BCG vaccination, and the ability to complete the test in a single visit [17]. The disadvantages include a potentially more complex interpretation and higher cost compared to TST [18]. Based on the Korean National Health Insurance reimbursement system, the official medical fee for IGRA is 585.1 points (approximately 40.5 USD), whereas that for TST is 62.6 points (approximately 4.3 USD), indicating that IGRA is nearly ten times more expensive than TST [19,20]. The antigens used in IGRA tests may vary slightly between manufacturers when using enzyme-linked immunosorbent assay (ELISA), but commonly include early secreted antigenic target 6 kDa (ESAT-6), culture filtrate protein 10 (CFP-10), and TB7.7 [21]. In the case of enzyme-linked immunospot (ELISPOT), ESAT-6 and CFP-10 are used [22]. For the QuantiFERON-TB Gold Plus kit (IGRA testing measured by ELISA), blood is collected into four tubes in the following critical order: Nil (negative control), TB1, TB2, and Mitogen (positive control) [21]. TB1 contains the long peptides ESAT-6 and CFP-10 that stimulate the CD4 + T-cell immune response. TB2 contains short peptides, in addition to the peptides in TB1 that elicit both CD4 and CD8 T cell immune responses [23]. In cases where the IGRA test result is positive, it is common for both TB1 and TB2 to simultaneously show positivity. However, there are instances where only TB2 is positive, which is likely due to a CD8 + T-cell response. Furthermore, it is also possible to observe a negative result upon follow-up testing [24]. It is impossible to directly and comprehensively compare all available kits, considering the heterogeneity of study design, different target populations, limited data among the newly developed kits, and the possibility of manipulations by the manufacturers.
In South Korea, commercialized IGRA that are currently in use include the QuantiFERON-TB Gold Plus (Qiagen) and T-SPOT.TB (Oxford Immunotec), Standard E TB-Feron Enzyme-Linked Immunosorbent Assay (SD Biosensor), Advansure TB-IGRA (Invitros), and the ichroma™ IGRA-TB (Boditech Med Inc.) [17,25,26]. In 2011, the WHO recommended the use of QuantiFERON-Gold (QFT-G), QuantiFERON-TB Gold In-Tube (QFT-GIT), and Oxford Immunotec T-SPOT.TB. In an online advisory meeting held in October 2021, the WHO concluded that Beijing Wantai’s TB-IGRA (Wantai) was comparable to that of the QuantiFERON-TB Gold Plus (QFT-Plus) [21]. However, for other products, the available studies are heterogeneous in terms of population, sample size, and methodology, and additional data are required, highlighting the need for continued comparative evaluation studies involving various groups.
T-SPOT.TB is an IGRA that detects the release of interferon-gamma (IFN-γ) from T-cells in response to M. tuberculosis-specific antigens measured using the ELISPOT [27]. The test involves separating peripheral blood mononuclear cells, washing them to remove potential interfering factors, and counting live T cells. This methodology aims to minimize indeterminate results, particularly in patients with compromised immune systems. Bae et al. [28] compared the sensitivity of QFT-GIT and T-SPOT.TB in elderly and immunocompromised groups. The overall sensitivity of QFT-GIT and T-SPOT.TB are 80.2% and 91.0%, respectively. Among the immunocompromised patients, the indeterminate results are significantly lower for T-SPOT.TB (2.4%) than QFT-GIT (13.1%) [28]. Another study by Redelman-Sidi et al. [29] evaluated the performance of IGRAs in diagnosing latent TB among immunocompromised adults, including individuals with HIV infection and those receiving corticosteroids. It was observed that T-SPOT.TB is less affected by low CD4+ T-cell counts than QFT-GIT, suggesting a potential advantage in certain immunocompromised populations [29]. In September 2020, the T-SPOT.TB received FDA premarket approval for the use of this test in individuals 2 years of age and older [30].
Age is related to the performance of latent TB testing. According to the 5th edition of the Korean guidelines for TB, it is recommended to use the TST for individuals under 5 years of age and either the TST or IGRA for those older than 5 years [31]. This is because the sensitivity of the IGRA is lower than that of the TST in children under 5 years of age. This reflects concerns that IGRA may have reduced sensitivity in very young children, particularly those under 2 years of age, and the limited evidence base in this group. Consistent with this, the American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention and American Academy of Pediatrics guidelines generally favor TST for young children (< 5 years and < 2 years, respectively) [32]. Similarly, Korean guidelines advise against IGRA as a first-line test in children younger than 5 years of age [31]. Nevertheless, exceptions have been recognized in the context of the BCG vaccination. If BCG is administered after 1 year of age or if a child received two or more BCG doses, Korean guidelines recommend IGRA even in children under 5 years of age [31]. This is because IGRA uses M. tuberculosis–specific antigens (ESAT-6 and CFP-10) that are absent in BCG strains; thus, IGRA is unaffected by prior BCG vaccination or by most environmental mycobacteria [33]. In contrast, TST relies on PPD, which shares antigens with BCG, leading to false-positive results [32]. Importantly, the effect of BCG on TST can persist for decades, and its impact is most pronounced in young children [34]. If TST and IGRA are performed simultaneously, blood collection for IGRA should be conducted on the day the TST is read (48-72 hours later) [35]. Performing IGRA for more than 4 days after TST administration can increase the likelihood of a positive IGRA result [31].
Research on the utility of the IGRA in children is ongoing. A meta-analysis reported that the sensitivity of IGRA in children under 18 years of age with microbiologically confirmed TB was between 88.5% and 89.6%, showing no significant difference compared to TST (88.2%). In contrast, the IGRA (exceeded 95.0%) has a significantly higher specificity than that of the TST (86.3%) [36]. In another study, the sensitivity of IGRA in children, aged 5 to 18 years, was 95.7%, which was significantly higher than that of the TST (82.6%) [37]. Further research is required to evaluate the utility of the IGRA in children. In a retrospective study conducted in the United States on external arrivals, such as TB contacts, immigrants from endemic countries, and refugees, children under two years of age showed a positive response to mitogens, suggesting that IGRA could assist in diagnosing latent TB in this age group. However, only 8 of 116 individuals received anti-TB treatment in this study; therefore, sensitivity and specificity were not assessed [38].
The effect of other vaccines on latent TB testing is currently being investigated. Although there are no definitive data on whether live vaccines affect latent TB testing, concerns have been raised that an anergic state induced by vaccination could lead to false-negative TST results. The TST and live vaccines can be administered simultaneously on the same day. However, considering the potential impact, it is recommended to wait for 4 weeks after a live vaccine before performing the TST [39]. The same applies to IGRA [40]. One study investigated the effect of coronavirus disease 2019 vaccines on IGRAs. This study included 134 individuals who received either the AstraZeneca-Oxford ChAdOx1 vaccine or the Moderna mRNA-1273 vaccine [41]. QuantiFERON-TB Gold Plus results were compared before and after the vaccination. The results showed an increase in both nil and mitogen values post-vaccination; however, this increase did not significantly affect the interpretation of the IGRA results [41].
Indeterminate IGRA test results are a frequent challenge in the laboratory. The indeterminate IGRA results indicate that the test could not reliably determine the presence or absence of M. tuberculosis infection [42]. By definition, these results arise when the assay fails to meet criteria for either a positive or negative outcome, typically due to an insufficient IFN-γ response to the mitogen control or high background inferences in the negative control. Extra care is necessary when interpreting these results, particularly for immunocompromised individuals. In a meta-analysis of 28 studies involving 14,792 immunocompromised individuals, the risk of active TB among those with indeterminate results was found to be low, with a risk ratio of 0.5 (95% confidence interval [CI], 0.3-0.8), when compared to positive results. However, the risk was significantly high, with a risk ratio of 2.9 (95% CI, 1.8-4.9), when compared to negative results. Taken together, these suggest that indeterminate results could be clinically significant [43]. Moreover, in immunocompromised patients, the incidence of indeterminate latent TB testing results may be elevated. Conditions which are managed by immunosuppression, such as HIV infection, chronic kidney disease, organ transplantation, have been the focus of current research. Based on clinical significance, particular attention should be paid to individuals with decreased renal function (2.9%–9.7%), inflammatory conditions, specific systemic autoimmune rheumatic diseases (such as inflammatory bowel disease [0.6%–22%]), and complex physiological states associated with dynamic inflammatory responses (such as pregnancy [13%–16%] and the postpartum period [0%–1%]) [44–51]. The risk factors contributing to indeterminate outcomes of QuantiFERON-TB Gold Assays are summarized in Table 1.
Table 1. Indeterminate results from QuantiFERON-TB Gold Assays in different populations
| Author | Study period | Region | Sample size | Target disease | Indeterminate rate | Risk factor |
|---|---|---|---|---|---|---|
| Ogawa et al. [44] | 2016–2017 | Japan | 118 | Hemodialysis | 5.9% | Long-term hemodialysis, older age (≥ 60 years) |
| Edathodu et al. [45] | 2008–2013 | Saudi Arabia | 278 | ESRD, pre- and post-transplant | 2.9% | Use of immunosuppressants, ESRD |
| Kim et al. [46] | 2010–2013 | South Korea | 784 | Kidney and pancreas transplant | 9.7% | Use of immunosuppressants |
| Stevens et al. [47] | 2011–2017 | USA (Georgia) | 859 | Pediatric IBD | Diagnosis: 10.3%, Treatment: 5.3% | High disease activity, malnutrition |
| Kurti et al. [48] | 2013–2014 | Hungary | 166 | IBD (Only stable patients included) | 0.6% | Smoking, use of immunosuppressants |
| Hakimian et al. [49] | 2008–2016 | USA (Massachusetts) | IBD: 107, RA: 89 | IBD, RA | IBD: 22%, RA: 7% | High-dose steroids (≥ 20 mg), elevated ESR/CRP |
| Weinberg et al. [50] | 2020 | Multinational (Africa, Asia, Caribbean) | 944 | Pregnant women with HIV | Pregnancy: 13%, Postpartum: 1% | Low CD4+ T-cell count (≤ 350 cells/µL), pregnancy-induced immunosuppression |
| LaCourse et al. [51] | 2014–2015 | Kenya | 100 | Pregnant women with HIV | Pregnancy: 16%, Postpartum: 0% | Pregnancy-related immune changes, low CD4+ T-cell count |
Abbreviations: ESRD, end-stage renal disease; IBD, inflammatory bowel disease; RA, rheumatoid arthritis; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; HIV, human immunodeficiency virus.
The interpretation is less straightforward in immunocompetent individuals. While indeterminate results still indicate that the latent TB status cannot be defined, there are no universally accepted guidelines for follow-up. In clinical practice, repeating the IGRA or performing a TST may be considered [52]. Meta-analyses indicate that individuals with indeterminate results have an intermediate risk of progression to active TB, which is higher than that in those with negative tests but lower than that in those with positive tests [43]. In such cases, repeat testing or adjunctive diagnostic evaluation (e.g., chest imaging and clinical risk stratification) may be warranted to refine clinical decision-making [31].
Isoniazid and rifampicin can be administered to individuals diagnosed with latent TB. According to the Korean Disease Control and Prevention Agency’s report on the 2017-2018 national latent TB screening project for workers in group facilities, active treatment has been reported to prevent 80%-90% of active TB cases. However, because the follow up was relatively brief (2.24 years), future long-term follow-up studies are necessary [13]. Another study involving 1,120,948 participants from the Korean National Health Screening Program between 2017 and 2020 indicated that the IGRA-positive/untreated group had 121.4 cases per 100,000 person-years compared with 23.6 cases in the IGRA-positive/treated group, highlighting the benefits of active treatment. Based on interferon levels from IGRA test results, the hazard ratio was 6.56 for the Q1 (0.35–0.67 IU/mL) group and 31.81 for the Q4 (3.33–10 IU/mL) group, a difference of approximately 4.9 times [53]. These findings are valuable for developing efficient screening and treatment plans for latent TB. These results underscore the utility of IGRA for risk stratification of latent TB, although it should not be used for monitoring treatment response or for diagnosing relapse or reinfection after the completion of TB therapy.
Latent TB represents a continuum, ranging from incipient to subclinical and active, affecting approximately 25% of the global population. TST and IGRA are used for diagnosis, although both tests cannot be used to differentiate infection stages. Moreover, these tests cannot be used to monitor treatment responses. Nevertheless, IGRA offers higher specificity and is unaffected by BCG vaccination, but is more expensive and more likely to yield indeterminate results, particularly among immunocompromised individuals or those with physiologically dynamic states, such as renal dysfunction, autoimmune disease, or pregnancy. Indeterminate results are clinically significant with a higher risk of progression than negative tests. Importantly, treatment of latent TB reduces the risk of active TB by up to 80%–90%. Overall, IGRA-based risk stratification aids in identifying high-risk groups, while also emphasizing the ongoing need to develop complementary methods for treatment monitoring or diagnosing relapse/reinfection.
This was not a human population study; therefore, approval by the Institutional Review Board or informed consent was not required.
No potential conflicts of interest relevant to this article were reported.
None.
This review article does not involve the generation or analysis of new datasets. All data supporting the findings are derived from previously published studies, which are appropriately cited within the manuscript.
1. WHO. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization; 2018.
2. Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018;31:e00021-18.


3. Cohen A, Mathiasen VD, Schon T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 2019;54:1900655.

4. Han S, Park Y, Kim J, Lee H, Cho H. Results of the tuberculosis epidemiological investigation in congregated settings, 2022. Public Health Wkly Rep 2023;16:950-64.
5. Lee S, Lee YL, Kim YC, Kim EJ, Heo JY, Choi YH. Prevalence and risk factors of latent tuberculosis infection among healthcare workers. Korean J Healthc Assoc Infect Control Prev 2019;24:52-9.
6. Lv H, Wang L, Zhang X, Dang C, Liu F, Zhang X, et al. Further analysis of tuberculosis in eight high-burden countries based on the Global Burden of Disease Study 2021 data. Infect Dis Poverty 2024;13:70.


7. Ai JW, Ruan QL, Liu QH, Zhang WH. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 2016;5:e10.


8. Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020;69:1-11.


9. Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ 2020;368:m549.


10. Zellweger JP. Latent tuberculosis infection in rural China: who will develop tuberculosis? Lancet Infect Dis 2017;17:1007-8.

11. Blount RJ, Tran MC, Everett CK, Cattamanchi A, Metcalfe JZ, Connor D, et al. Tuberculosis progression rates in U.S. immigrants following screening with interferon-gamma release assays. BMC Public Health 2016;16:875.


12. Lee S, Lee JE, Kang JS, Lee SO, Lee SH. Long-term performance of the IGRA to predict and prevent active tuberculosis development in HIV-infected patients. Int J Tuberc Lung Dis 2019;23:422-7.

13. Kim HW, Min JS, Kim JS, Kim GH, Jeon CM, In HK, et al. Analysis of active tuberculosis incidence in the national latent tuberculosis infection cohort. Public Health Wkly Rep 2020;13:1130-47.
14. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin-past, present, and future. FEMS Immunol Med Microbiol 2012;66:273-80.


15. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:111-5.


16. Kobashi Y. Current status and future landscape of diagnosing tuberculosis infection. Respir Investig 2023;61:563-78.

17. Hur YG, Hong JY, Choi DH, Kim A, Park SY, Kwon M, et al. A feasibility study for diagnosis of latent tuberculosis infection using an IGRA point-of-care platform in South Korea. Yonsei Med J 2019;60:375-80.


18. Zhang B, Xu Y, Huang Z, Li R, Zhu T, Liang S, et al. Consolidated microscale interferon-γ release assay with tip optofluidic immunoassay for dynamic parallel diagnosis of tuberculosis infection. Anal Chem 2025;97:2863-72.

19. Cho H, Seok J, Park Y, Kim HJ, Lee EH, Park J, et al. Cost-effectiveness of age-expanding strategy of latent tuberculosis infection treatment in household contacts in South Korea. Yonsei Med J 2023;64:366-74.


20. Health Insurance Review and Assessment Service (HIRA). Medical care benefit costs under national health insurance. https://biz.hira.or.kr/index.do [Online] (last visited on 26 August 2025).
21. WHO. The use of alternative commercial IGRAs for the detection of TB infection: policy statement. Geneva: World Health Organization; 2021.
22. Hill PC, Jackson-Sillah D, Fox A, Franken KL, Lugos MD, Jeffries DJ, et al. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J Clin Microbiol 2005;43:2070-4.


23. QIAGEN. QuantiFERON®-TB Gold Plus ELISA kit instructions for use. Hilden, Germany: QIAGEN; 2023.
24. Shin E, Seo JD, Kim H, Hur M, Yun YM, Moon HW. Analysis of positive patterns and follow-up results of the QuantiFERON-TB Gold-Plus test in the screening of patients and healthcare workers. Lab Med Online 2023;13:85-90.
25. Kweon OJ, Lim YK, Kim HR, Kim T, Lee M. Evaluation of Standard E TB-Feron Enzyme-Linked Immunosorbent Assay for diagnosis of latent tuberculosis infection in health care workers. J Clin Microbiol 2019;57:10.1128/jcm.01347-19.


26. Kim JJ, Park Y, Choi D, Kim HS. Performance evaluation of a new automated chemiluminescent immunoanalyzer-based interferon-gamma releasing assay AdvanSure I3 in comparison with the QuantiFERON-TB Gold In-Tube assay. Ann Lab Med 2020;40:33-9.


27. Kim HS, Kim CH, Hur M, Hyun IG, Park MJ, Song W, et al. Clinical usefulness of T-SPOT.TB test for the diagnosis of tuberculosis. Korean J Lab Med 2010;30:171-7.

28. Bae W, Park KU, Song EY, Kim SJ, Lee YJ, Park JS, et al. Comparison of the sensitivity of QuantiFERON-TB Gold In-Tube and T-SPOT.TB according to patient age. PLoS One 2016;11:e0156917.


29. Redelman-Sidi G and Sepkowitz KA. IFN-gamma release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med 2013;188:422-31.


30. U.S. Food and Drug Administration. T-SPOT.TB Premarket Approval (PMA number: P070006/S014). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P070006S014 [Online] (last visited on 30 June 2025).
31. The Korean Academy of Tuberculosis and Respiratory Diseases. Korean guidelines for tuberculosis. 5th ed. Seoul: Korea Centers for Disease Control and Prevention; 2024.
32. Turner NA, Ahmed A, Haley CA, Starke JR, Stout JE. Use of interferon-gamma release assays in children <2 years old. J Pediatric Infect Dis Soc 2023;12:481-5.

33. Flood J and Wendorf KA. Mounting evidence for IFN-γ release assay use in young children. Am J Respir Crit Care Med 2018;197:983-5.

34. Diel R. Long-term effect of bacille Calmette-Guerin vaccination in tuberculin skin testing: a new reality for TB prevention. Chest 2017;152:235-6.

35. Song S, Jeon D, Kim JW, Kim YD, Kim SP, Cho JS, et al. Performance of confirmatory interferon-γ release assays in school TB outbreaks. Chest 2012;141:983-8.

36. Laurenti P, Raponi M, de Waure C, Marino M, Ricciardi W, Damiani G. Performance of interferon-γ release assays in the diagnosis of confirmed active tuberculosis in immunocompetent children: a new systematic review and meta-analysis. BMC Infect Dis 2016;16:131.


37. Kay AW, Islam SM, Wendorf K, Westenhouse J, Barry PM. Interferon-γ release assay performance for tuberculosis in childhood. Pediatrics 2018;141: e20173918.

38. Gaensbauer J, Young J, Harasaki C, Aiona K, Belknap R, Haas MK. Interferon-gamma release assay testing in children younger than 2 years in a US-based health system. Pediatr Infect Dis J 2020;39:803-7.

39. Wharton M, Strikas RA, Harpaz R, Rotz LD, Schwartz B, Casey CG, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003;52:1-16.
40. Centers for Disease Control and Prevention. Special situations – general best practice guidelines for immunization. Vaccines & immunizations. https://www.cdc.gov/vaccines/hcp/imz-best-practices/special-situations.html [Online] (last visited on 30 June 2025).
41. Chen NY, Liu ZH, Kao SW, Lin HS, Lee IK, Zheng JY, et al. Changes in interferon- γ release assay readout after COVID-19 vaccination: a prospective cohort study. Int J Infect Dis 2022;122:537-42.


42. Palanivel J, Sounderrajan V, Thangam T, Rao SS, Harshavardhan S, Parthasarathy K. Latent tuberculosis: challenges in diagnosis and treatment, perspectives, and the crucial role of biomarkers. Curr Microbiol 2023;80:392.

43. Zhou G, Luo S, He J, Chen N, Zhang Y, Cai S, et al. Risk of progression to active tuberculosis for indeterminate interferon-gamma release assay in immunocompromised individuals: a systematic review and meta-analysis. Clin Microbiol Infect 2023;29:1375-84.

44. Ogawa Y, Harada M, Hashimoto K, Kamijo Y. Prevalence of latent tuberculosis infection and its risk factors in Japanese hemodialysis patients. Clin Exp Nephrol 2021;25:1255-65.

45. Edathodu J, Varghese B, Alrajhi AA, Shoukri M, Nazmi A, Elgamal H, et al. Diagnostic potential of interferon-gamma release assay to detect latent tuberculosis infection in kidney transplant recipients. Transpl Infect Dis 2017;19:e12675.

46. Kim SH, Lee SO, Park IA, Kim SM, Park SJ, Yun SC, et al. Isoniazid treatment to prevent TB in kidney and pancreas transplant recipients based on an interferon-γ-releasing assay: an exploratory randomized controlled trial. J Antimicrob Chemother 2015;70:1567-72.

47. Stevens JP, Ballengee CR, Chandradevan R, Thompson AB, Schoen BT, Kugathasan S, et al. Performance of interferon-gamma release assays for tuberculosis screening in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019;69:e162.
48. Kurti Z, Lovasz BD, Gecse KB, Balint A, Farkas K, Morocza-Szabo A, et al. Tuberculin skin test and Quantiferon in BCG vaccinated, immunosuppressed patients with moderate-to-severe inflammatory bowel disease. J Gastrointestin Liver Dis 2015;24:467-72.

49. Hakimian S, Popov Y, Rupawala AH, Salomon-Escoto K, Hatch S, Pellish R. The conundrum of indeterminate QuantiFERON-TB Gold results before anti-tumor necrosis factor initiation. Biologics 2018;12:61-7.


50. Weinberg A, Aaron L, Montepiedra G, Sterling TR, Browning R, Mmbaga B, et al. Effects of pregnancy and isoniazid preventive therapy on Mycobacterium tuberculosis interferon gamma response assays in women with HIV. Clin Infect Dis 2021;73:e3555-e62.


51. LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, Horne DJ, et al. Effect of pregnancy on interferon gamma release assay and tuberculin skin test detection of latent TB infection among HIV-infected women in a high burden setting. J Acquir Immune Defic Syndr 2017;75:128-36.


52. Campbell JR, Pease C, Daley P, Pai M, Menzies D. Chapter 4: diagnosis of tuberculosis infection. Can J Respir Crit Care Sleep Med 2022;6(sup1):49-65.
53. Kim HW, Min J, Kim JS, Park Y, Kim Y, Kim G, et al. Tuberculosis risk and efficacy of latent tuberculosis infection treatment among participants of national latent tuberculosis infection screening program. Public Health Wkly Rep 2024;17:1314-34.
1. WHO. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization; 2018.
2. Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018;31:e00021-18.


3. Cohen A, Mathiasen VD, Schon T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 2019;54:1900655.

4. Han S, Park Y, Kim J, Lee H, Cho H. Results of the tuberculosis epidemiological investigation in congregated settings, 2022. Public Health Wkly Rep 2023;16:950-64.
5. Lee S, Lee YL, Kim YC, Kim EJ, Heo JY, Choi YH. Prevalence and risk factors of latent tuberculosis infection among healthcare workers. Korean J Healthc Assoc Infect Control Prev 2019;24:52-9.
6. Lv H, Wang L, Zhang X, Dang C, Liu F, Zhang X, et al. Further analysis of tuberculosis in eight high-burden countries based on the Global Burden of Disease Study 2021 data. Infect Dis Poverty 2024;13:70.


7. Ai JW, Ruan QL, Liu QH, Zhang WH. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 2016;5:e10.


8. Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020;69:1-11.


9. Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ 2020;368:m549.


10. Zellweger JP. Latent tuberculosis infection in rural China: who will develop tuberculosis? Lancet Infect Dis 2017;17:1007-8.

11. Blount RJ, Tran MC, Everett CK, Cattamanchi A, Metcalfe JZ, Connor D, et al. Tuberculosis progression rates in U.S. immigrants following screening with interferon-gamma release assays. BMC Public Health 2016;16:875.


12. Lee S, Lee JE, Kang JS, Lee SO, Lee SH. Long-term performance of the IGRA to predict and prevent active tuberculosis development in HIV-infected patients. Int J Tuberc Lung Dis 2019;23:422-7.

13. Kim HW, Min JS, Kim JS, Kim GH, Jeon CM, In HK, et al. Analysis of active tuberculosis incidence in the national latent tuberculosis infection cohort. Public Health Wkly Rep 2020;13:1130-47.
14. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin-past, present, and future. FEMS Immunol Med Microbiol 2012;66:273-80.


15. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:111-5.


16. Kobashi Y. Current status and future landscape of diagnosing tuberculosis infection. Respir Investig 2023;61:563-78.

17. Hur YG, Hong JY, Choi DH, Kim A, Park SY, Kwon M, et al. A feasibility study for diagnosis of latent tuberculosis infection using an IGRA point-of-care platform in South Korea. Yonsei Med J 2019;60:375-80.


18. Zhang B, Xu Y, Huang Z, Li R, Zhu T, Liang S, et al. Consolidated microscale interferon-γ release assay with tip optofluidic immunoassay for dynamic parallel diagnosis of tuberculosis infection. Anal Chem 2025;97:2863-72.

19. Cho H, Seok J, Park Y, Kim HJ, Lee EH, Park J, et al. Cost-effectiveness of age-expanding strategy of latent tuberculosis infection treatment in household contacts in South Korea. Yonsei Med J 2023;64:366-74.


20. Health Insurance Review and Assessment Service (HIRA). Medical care benefit costs under national health insurance. https://biz.hira.or.kr/index.do [Online] (last visited on 26 August 2025).
21. WHO. The use of alternative commercial IGRAs for the detection of TB infection: policy statement. Geneva: World Health Organization; 2021.
22. Hill PC, Jackson-Sillah D, Fox A, Franken KL, Lugos MD, Jeffries DJ, et al. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J Clin Microbiol 2005;43:2070-4.


23. QIAGEN. QuantiFERON®-TB Gold Plus ELISA kit instructions for use. Hilden, Germany: QIAGEN; 2023.
24. Shin E, Seo JD, Kim H, Hur M, Yun YM, Moon HW. Analysis of positive patterns and follow-up results of the QuantiFERON-TB Gold-Plus test in the screening of patients and healthcare workers. Lab Med Online 2023;13:85-90.
25. Kweon OJ, Lim YK, Kim HR, Kim T, Lee M. Evaluation of Standard E TB-Feron Enzyme-Linked Immunosorbent Assay for diagnosis of latent tuberculosis infection in health care workers. J Clin Microbiol 2019;57:10.1128/jcm.01347-19.


26. Kim JJ, Park Y, Choi D, Kim HS. Performance evaluation of a new automated chemiluminescent immunoanalyzer-based interferon-gamma releasing assay AdvanSure I3 in comparison with the QuantiFERON-TB Gold In-Tube assay. Ann Lab Med 2020;40:33-9.


27. Kim HS, Kim CH, Hur M, Hyun IG, Park MJ, Song W, et al. Clinical usefulness of T-SPOT.TB test for the diagnosis of tuberculosis. Korean J Lab Med 2010;30:171-7.

28. Bae W, Park KU, Song EY, Kim SJ, Lee YJ, Park JS, et al. Comparison of the sensitivity of QuantiFERON-TB Gold In-Tube and T-SPOT.TB according to patient age. PLoS One 2016;11:e0156917.


29. Redelman-Sidi G and Sepkowitz KA. IFN-gamma release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med 2013;188:422-31.


30. U.S. Food and Drug Administration. T-SPOT.TB Premarket Approval (PMA number: P070006/S014). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P070006S014 [Online] (last visited on 30 June 2025).
31. The Korean Academy of Tuberculosis and Respiratory Diseases. Korean guidelines for tuberculosis. 5th ed. Seoul: Korea Centers for Disease Control and Prevention; 2024.
32. Turner NA, Ahmed A, Haley CA, Starke JR, Stout JE. Use of interferon-gamma release assays in children <2 years old. J Pediatric Infect Dis Soc 2023;12:481-5.

33. Flood J and Wendorf KA. Mounting evidence for IFN-γ release assay use in young children. Am J Respir Crit Care Med 2018;197:983-5.

34. Diel R. Long-term effect of bacille Calmette-Guerin vaccination in tuberculin skin testing: a new reality for TB prevention. Chest 2017;152:235-6.

35. Song S, Jeon D, Kim JW, Kim YD, Kim SP, Cho JS, et al. Performance of confirmatory interferon-γ release assays in school TB outbreaks. Chest 2012;141:983-8.

36. Laurenti P, Raponi M, de Waure C, Marino M, Ricciardi W, Damiani G. Performance of interferon-γ release assays in the diagnosis of confirmed active tuberculosis in immunocompetent children: a new systematic review and meta-analysis. BMC Infect Dis 2016;16:131.


37. Kay AW, Islam SM, Wendorf K, Westenhouse J, Barry PM. Interferon-γ release assay performance for tuberculosis in childhood. Pediatrics 2018;141: e20173918.

38. Gaensbauer J, Young J, Harasaki C, Aiona K, Belknap R, Haas MK. Interferon-gamma release assay testing in children younger than 2 years in a US-based health system. Pediatr Infect Dis J 2020;39:803-7.

39. Wharton M, Strikas RA, Harpaz R, Rotz LD, Schwartz B, Casey CG, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003;52:1-16.
40. Centers for Disease Control and Prevention. Special situations – general best practice guidelines for immunization. Vaccines & immunizations. https://www.cdc.gov/vaccines/hcp/imz-best-practices/special-situations.html [Online] (last visited on 30 June 2025).
41. Chen NY, Liu ZH, Kao SW, Lin HS, Lee IK, Zheng JY, et al. Changes in interferon- γ release assay readout after COVID-19 vaccination: a prospective cohort study. Int J Infect Dis 2022;122:537-42.


42. Palanivel J, Sounderrajan V, Thangam T, Rao SS, Harshavardhan S, Parthasarathy K. Latent tuberculosis: challenges in diagnosis and treatment, perspectives, and the crucial role of biomarkers. Curr Microbiol 2023;80:392.

43. Zhou G, Luo S, He J, Chen N, Zhang Y, Cai S, et al. Risk of progression to active tuberculosis for indeterminate interferon-gamma release assay in immunocompromised individuals: a systematic review and meta-analysis. Clin Microbiol Infect 2023;29:1375-84.

44. Ogawa Y, Harada M, Hashimoto K, Kamijo Y. Prevalence of latent tuberculosis infection and its risk factors in Japanese hemodialysis patients. Clin Exp Nephrol 2021;25:1255-65.

45. Edathodu J, Varghese B, Alrajhi AA, Shoukri M, Nazmi A, Elgamal H, et al. Diagnostic potential of interferon-gamma release assay to detect latent tuberculosis infection in kidney transplant recipients. Transpl Infect Dis 2017;19:e12675.

46. Kim SH, Lee SO, Park IA, Kim SM, Park SJ, Yun SC, et al. Isoniazid treatment to prevent TB in kidney and pancreas transplant recipients based on an interferon-γ-releasing assay: an exploratory randomized controlled trial. J Antimicrob Chemother 2015;70:1567-72.

47. Stevens JP, Ballengee CR, Chandradevan R, Thompson AB, Schoen BT, Kugathasan S, et al. Performance of interferon-gamma release assays for tuberculosis screening in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019;69:e162.
48. Kurti Z, Lovasz BD, Gecse KB, Balint A, Farkas K, Morocza-Szabo A, et al. Tuberculin skin test and Quantiferon in BCG vaccinated, immunosuppressed patients with moderate-to-severe inflammatory bowel disease. J Gastrointestin Liver Dis 2015;24:467-72.

49. Hakimian S, Popov Y, Rupawala AH, Salomon-Escoto K, Hatch S, Pellish R. The conundrum of indeterminate QuantiFERON-TB Gold results before anti-tumor necrosis factor initiation. Biologics 2018;12:61-7.


50. Weinberg A, Aaron L, Montepiedra G, Sterling TR, Browning R, Mmbaga B, et al. Effects of pregnancy and isoniazid preventive therapy on Mycobacterium tuberculosis interferon gamma response assays in women with HIV. Clin Infect Dis 2021;73:e3555-e62.


51. LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, Horne DJ, et al. Effect of pregnancy on interferon gamma release assay and tuberculin skin test detection of latent TB infection among HIV-infected women in a high burden setting. J Acquir Immune Defic Syndr 2017;75:128-36.


52. Campbell JR, Pease C, Daley P, Pai M, Menzies D. Chapter 4: diagnosis of tuberculosis infection. Can J Respir Crit Care Sleep Med 2022;6(sup1):49-65.
53. Kim HW, Min J, Kim JS, Park Y, Kim Y, Kim G, et al. Tuberculosis risk and efficacy of latent tuberculosis infection treatment among participants of national latent tuberculosis infection screening program. Public Health Wkly Rep 2024;17:1314-34.