Ann Clin Microbiol 2025;28(3):14. https://doi.org/10.5145/ACM.2025.28.3.3
Received on 15 May 2025, Revised on 5 August 2025, Accepted on 5 August 2025, Published on 20 September 2025.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Background: Accurate diagnosis of Clostridioides difficile infection (CDI) requires both microbiologic confirmation and clinical correlation. Current guidelines recommend a two-step algorithm combining a sensitive screening test with a specific confirmatory assay. This study evaluated the diagnostic performance of the glutamate dehydrogenase (GDH)/toxin enzyme immunoassay (EIA) over five years and assessed its suitability as an initial screening test.
Methods: We retrospectively analyzed 8,685 C. difficile-related tests conducted between March 2020 and February 2025. The GDH/toxin EIA was performed using the C. DIFF QUIK CHEK COMPLETE (TechLab). Toxigenic culture involved alcohol-shocked stool samples plated on chromogenic agar and incubated anaerobically for 48 hours. Toxin gene polymerase chain reaction (PCR) was done using the BD MAX Cdiff assay and the Xpert C. difficile assay.
Results: The GDH test showed a sensitivity of 77.0% and negative predictive value (NPV) of 95.1% compared with culture. The toxin EIA showed 35.0% sensitivity and 96.9% positive predictive value relative to PCR. The combined GDH+Toxin EIA achieved 82.6% sensitivity and 96.9% NPV compared with PCR. Most discordant results involved low bacterial burden or non-toxigenic isolates. GDH positivity correlated with growth quantity, and toxin EIA positivity varied by ribotype. Algorithm modeling suggested the GDH/toxin test as a cost-effective first-line option.
Conclusion: The GDH/toxin EIA demonstrated high NPV and may be appropriate as an initial test in CDI diagnostic algorithms. These findings support its role in diagnostic stewardship and provide evidence to inform the development of national diagnostic guidelines in Korea.
Algorithms, Clostridioides difficile, Clostridioides difficile infections, Glutamate dehydrogenase, Toxin
Clostridioides difficile infection (CDI) is becoming increasingly prevalent worldwide, primarily due to the rise in antibiotic use and an aging population. However, the optimal diagnostic strategy remains debatable [1]. Accurate diagnosis requires both microbiological confirmation and clinical assessment. Microbiological confirmation includes detection of the organism and its toxin production, while clinical assessment involves factors such as recent antibiotic use, exclusion of other causes of diarrhea, and consideration of asymptomatic colonization. Clinical guidelines play a crucial role in supporting appropriate diagnostic practices.
Major guidelines, including those from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [2], Infectious Diseases Society of America, Society for Healthcare Epidemiology of America [3], and American College of Gastroenterology (ACG) [4], recommend testing algorithms rather than relying on a single diagnostic test.
The 2016 updated ESCMID guidelines [2] recommend a two-step testing algorithm that combines a highly sensitive test with a highly specific test for optimal CDI diagnostic accuracy. Two approaches were proposed. First, an initial screening with a highly sensitive assay, such as the nucleic acid amplification test (NAAT) or glutamate dehydrogenase (GDH) test, is followed by a more specific toxin A/B enzyme immunoassay (EIA). If the initial test result is negative, no further testing is required, and CDI is considered to be unlikely. If the initial test is positive, toxin EIA is performed; a positive result confirms CDI, whereas a negative result requires clinical judgment to assess potential colonization or infection with undetectable toxin levels. Given that no diagnostic test is perfectly sensitive, the final diagnosis and treatment decisions must rely on comprehensive clinical evaluation.
The second approach involves simultaneous testing of GDH and toxin A/B EIA. If both results are positive or negative, CDI can be diagnosed or excluded without further testing. In cases where GDH is positive but the toxin result is negative, additional testing with NAAT or toxigenic culture is recommended. If the follow-up test is negative, CDI is unlikely. If positive, clinical interpretation is required to distinguish between true infection and asymptomatic carriers. The ACG guidelines also endorse the first approach [4].
Sequential testing is rarely performed at our institution. Instead, clinicians frequently request multiple tests simultaneously, primarily for faster turnaround time and convenience. While GDH/toxin EIA is available daily, polymerase chain reaction (PCR) and C. difficile cultures are limited to weekdays. As a result, discordant results—such as GDH(−)/toxin(−) but PCR(+)—are occasionally encountered. These discordant results raise questions about the applicability of the recommended two-step algorithm in our clinical setting. They can complicate clinical decision-making, particularly when the PCR is positive despite negative results on initial screening tests, potentially leading to overtreatment or misclassification of colonization as infection.
Simultaneous testing may improve turnaround time but may also increase diagnostic cost and complicate interpretation when results conflict. Incorporating institutional data, we found that 7.2% of tested cases showed discordant results, many of which involved low bacterial burden, highlighting the complexity of interpretation in real-world settings.
This study aimed to evaluate the performance of the GDH/toxin EIA for C. difficile detection. We analyzed data from the past five years and assessed the assay’s suitability as an initial test within a two-step testing algorithm, as recommended by current clinical guidelines for CDI diagnosis.
This retrospective study evaluated the diagnostic accuracy, adhering to the Standards for Reporting Diagnostic Accuracy Studies guidelines (https://www.equator-network.org/reporting-guidelines/stard/).
The analysis included 8,685 C. difficile-related tests conducted over five years, from March 2020 to February 2025.
GDH and Toxin A/B tests (hereafter referred to as the toxin test) were performed using C. DIFF QUIK CHEK COMPLETE assay (CD COMPLETE; TechLab) according to the manufacturer’s instructions.
Alcohol-shocked stool specimens were inoculated onto C. difficile chromogenic agar (Asan) and incubated at 37°C for 48 h in an anaerobic chamber (Don Whitley Scientific). Isolates were identified using the MALDI Biotyper MBT Smart system and MALDI-TOF MS Biotyper software (version 4.1; Bruker Daltonics). The toxin A (tcdA) and B (tcdB) genes of the isolated strains were detected by PCR, as previously described [5,6]. The primer pairs used were tcdA-F and tcdA-R for tcdA and NK104-NK105 for tcdB.
Prior to March 2021, C. difficile toxin B PCR was performed using the BD MAX Cdiff assay (Becton Dickinson and Company). An Xpert C. difficile assay (Cepheid) was performed following the manufacturer’s instructions.
For isolates obtained between March 2020 and January 2023, binary toxin gene PCR was performed alongside toxin A and B gene PCR [7]. The primer pairs used were cdtA pos-cdtA rev for cdtA and cdtB pos-cdtB rev for cdtB. Toxin gene-positive isolates were genotyped by PCR ribotyping [8]. This process was performed as previously described using the primers 5′-CTGGGGTGAAGTCGTAACAAGG-3′ (position 1445 to 1466 of the 16S rRNA gene) and 5′-GCGCCCTTTGTAGCTTGACC-3′ (position 20 to 1 of the 23S rRNA gene). PCR ribotyping patterns were visually compared, with patterns differing by at least one band classified as distinct ribotypes. Ribotype groups were designated using a combination of uppercase and lowercase letters and numbers.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using 2×2 contingency tables with either toxigenic culture or toxin B PCR as the reference standard. Statistical differences between groups (e.g., GDH and toxin positivity by ribotype) were evaluated using the Pearson’s chi-squared test. Statistical significance was set at P < 0.05. All statistical analyses were performed using Python (version 3.11), an open-source programming language.
Over a five-year period, 8,685 C. difficile-related specimens were examined. Of these, 8,274 specimens underwent GDH/toxin testing, 6,921 were tested for C. difficile toxin B using PCR, and 6,728 were cultured for C. difficile. Only 2.7% (221 samples) were tested exclusively using the GDH/toxin test, indicating the infrequent application of the two-step testing algorithm in practice. A total of 6,911 tests (including duplicates) incorporated both GDH/toxin and toxin B PCR. Of these, 5,215 were negative for both GDH/toxin and toxin B by PCR, whereas 377 were positive for both. Consequently, applying the two-step algorithm would have resulted in additional toxin B PCR testing for 80.9% (5,592/6,911) of the samples. In accordance with the clinical guidelines, testing for a cure is not recommended [3,4]. Therefore, repeat tests performed within one month of a positive CDI diagnosis were excluded from this analysis.
Among the 6,641 samples tested with both the GDH/toxin test and C. difficile culture, the GDH test demonstrated a sensitivity, specificity, PPV, and NPV of 77.0%, 98.7%, 92.9%, and 95.1%, respectively (Table 1).
Among the 6,119 samples tested with both the GDH/toxin test and C. difficile toxin B PCR, the toxin EIA showed a sensitivity, specificity, PPV, and NPV of 35.0%, 99.8%, 96.9%, and 89.9%, respectively. Although the sensitivity was notably low (35.0%), both specificity and PPV were very high (Table 2).
Using toxin B PCR as the reference standard, the GDH/toxin EIA test exhibited sensitivity, specificity, PPV, and NPV of 82.6%, 95.0%, 73.9%, and 96.9%, respectively. The high NPV (96.9%) suggests the reliability of this test in ruling out C. difficile infection (Table 3).
Table 1. Comparison between GDH test and C. difficile culture (N = 6,641)
| C. difficile culture | |||
|---|---|---|---|
| Growth | No growth | ||
| GDH | + | 933 | 71 |
| − | 279 | 5,358 | |
Sensitivity: 77.0%; Specificity: 98.7%; PPV: 92.9%; NPV: 95.1%
Abbreviations: GDH, glutamate dehydrogenase; PPV, positive predictive value; NPV, negative predictive value.
Table 2. Comparison between toxin EIA test and C. difficile toxin B PCR (N = 6,119)
| C. difficile toxin B PCR | |||
|---|---|---|---|
| + | − | ||
| Toxin A/B | + | 315 | 10 |
| − | 585 | 5,209 | |
Sensitivity: 35.0%; Specificity: 99.8%; PPV: 96.9%; NPV: 89.9%
Abbreviations: EIA, enzyme immunoassay; PCR, polymerase chain reaction; PPV, positive predictive value; NPV, negative predictive value.
Table 3. Comparison between GDH/toxin EIA test and C. difficile toxin B PCR (N = 6,119)
| C. difficile toxin B PCR | |||
|---|---|---|---|
| + | − | ||
| GDH + toxin A/B | + | 743 | 263 |
| − | 157 | 4,956 | |
Sensitivity: 82.6%; Specificity: 95.0%; PPV: 73.9%; NPV: 96.9%
Abbreviations: EIA, enzyme immunoassay; PCR, polymerase chain reaction; GDH, glutamate dehydrogenase; PPV, positive predictive value; NPV, negative predictive value.
The analysis of the 1,365 cases with discrepant results is summarized in Fig 1. Among the cases with discrepant results between GDH and C. difficile cultures, 279 were GDH(–) and culture(+). Of these, non-toxigenic isolates were identified in 123 cases (44.1%), whereas among the remaining 156 cases of toxigenic isolates, 129 (46.2%) exhibited only one colony forming unit (CFU) or a few colonies. Among the 27 (9.7%) cases with ‘some’ or ‘heavy’ growth, 3 (1.1%) had alternative causes of diarrhea (rotavirus, norovirus, or Crohn’s disease), 5 (1.8%) had no clinical impression of CDI, 2 (0.7%) showed positive GDH results on follow-up, 1 (0.4%) recovered without treatment (R/O CDI), and 16 (5.7%) had no identifiable explanation. Additionally, among 71 cases with GDH(+) and culture(–) results, PCR was performed on 60 cases, of which 18 (30%) tested positive and 42 (70%) tested negative.
Among the discrepant results between toxin EIA and C. difficile toxin B PCR, 10 cases showed toxin EIA(+) and PCR(–) results. Culture was performed in nine of these cases, and all yielded either no growth or non-toxigenic isolates. Conversely, 585 cases showed toxin EIA(–) and PCR(+) results, with cultures performed in 495 cases. Of these, 78 (15.8%) showed no growth or were non-toxigenic isolates, and among the remaining 417 cases with toxigenic isolates, 199 (40.2%) showed only 1 CFU to a few colonies. Cultures were performed in 147 of the 157 patients with GDH(−)/toxin(−) and C. difficile toxin B PCR-positive results. Of these, 43 (29.3%) showed no C. difficile growth or growth of non-toxigenic isolates, 85 (57.8%) showed growth of only one CFU to a few colonies, 2 (1.4%) had no clinical impression of CDI, 1 (0.7%) had an alternative cause of diarrhea (norovirus infection), 1 (0.7%) was positive on the follow-up GDH test, and the remaining 15 (10.2%) had no identifiable explanation.
Among the 263 patients with GDH (+)/ toxin (−) and C. difficile toxin B PCR-negative results, C. difficile cultures were performed for 230 patients. Of these, 154 showed growth of non-toxigenic isolates. There were 76 discrepant cases, including 34 with toxigenic isolate growth and 42 with no C. difficile growth.
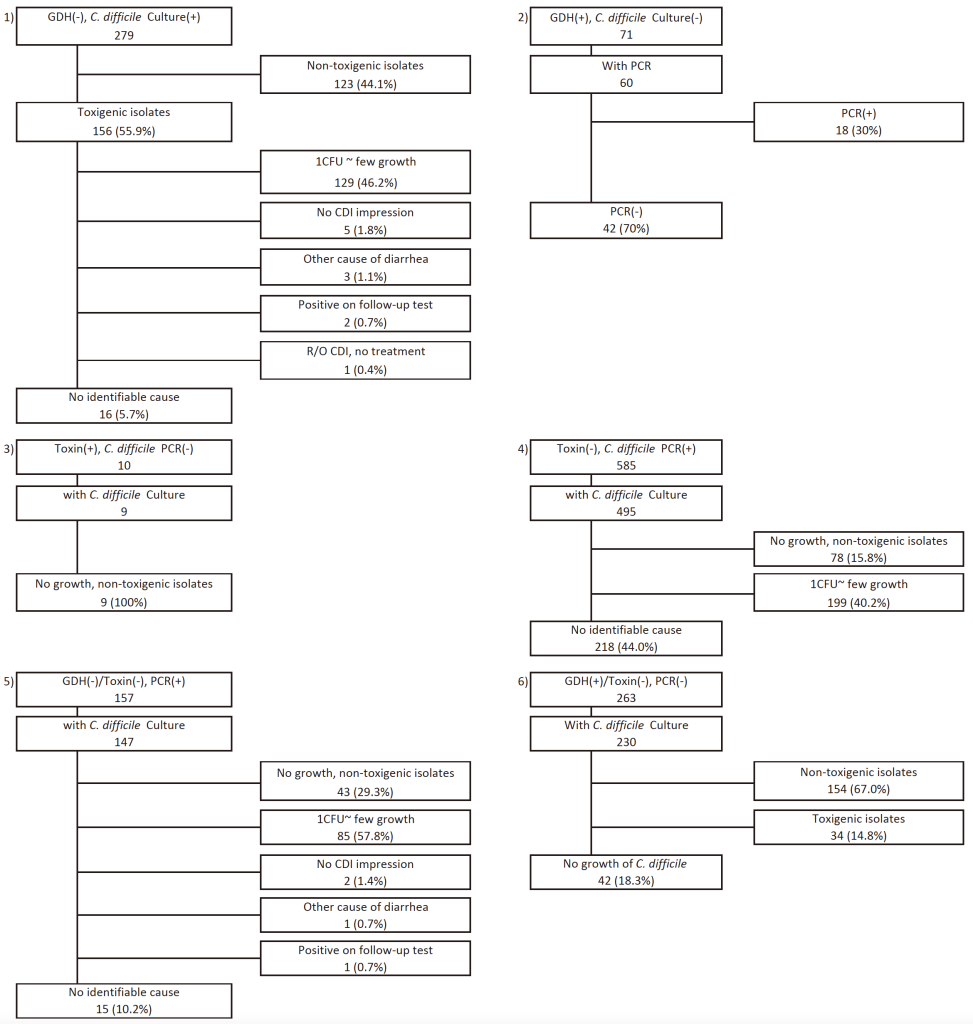
Fig. 1. Summary of discrepant cases between GDH/toxin, toxin B PCR, and C. difficile culture results.
From March 2020 to January 2023, 3,578 C. difficile cultures were analyzed, excluding duplicate isolates. Of these, 477 toxigenic (13.3%) and 182 non-toxigenic strains were identified. Non-toxigenic strains accounted for 27.6% of all C. difficile strains isolated during this period. The toxin statuses of the isolates were as follows: A+B+ (toxin A and B positive), 85.8%; A−B+ 4.8%; and A+B+CDT+ (binary toxin positive), 9.4%. Common ribotypes were R014/020 (18.7%), R002 (11.6%), R018 (8.7%), R106 (7.3%), R001 (5.2%), and R012 (5.2%).
Table 4 presents the GDH and toxin positivity rates according to the PCR ribotypes. Although GDH positivity rates did not differ significantly among ribotypes (P = 0.608), toxin EIA positivity rates differed significantly (P = 0.034).
Table 4. GDH and toxin A/B EIA positivity rates for C. difficile PCR ribotype
| Ribotypes | No. | GDH positive (%) | Toxin positive (%) |
|---|---|---|---|
| R014/020 | 78 | 77.9 | 30.8 |
| R002 | 50 | 80.0 | 38.0 |
| R018 | 37 | 86.5 | 59.5 |
| R106 | 33 | 90.9 | 48.5 |
| R012 | 23 | 78.3 | 13.0 |
| R001 | 23 | 82.3 | 30.4 |
| R046 | 18 | 88.9 | 33.3 |
| AB24 | 17 | 93.8 | 31.3 |
| AB25 | 16 | 94.1 | 35.3 |
| R159 | 15 | 86.7 | 26.7 |
| R078 | 12 | 91.7 | 58.3 |
| R017 | 9 | 100.0 | 55.6 |
| R070 | 9 | 77.8 | 44.4 |
| R023 | 5 | 60.0 | 60.0 |
| R369 | 7 | 85.7 | 57.1 |
| R027 | 4 | 100.0 | 75.0 |
| Other | 69 | 87.0 | 47.8 |
Abbreviations: GDH, glutamate dehydrogenase; EIA, enzyme immunoassay; PCR, polymerase chain reaction.
A comparison of C. difficile growth, toxin production, and GDH positivity is shown in Table 5. The GDH positivity rate increased with the degree of bacterial growth, exceeding 90% in isolates showing “some” or “many” colonies, regardless of the toxin production. In contrast, isolates with only one CFU exhibited notably lower GDH positivity rate: 42.9% for toxigenic strains and 31.6% for non-toxigenic strains.
Table 5. Comparison of C. difficile growth in cultured isolates and toxin production status with GDH positivity rate
| Bacterial growth | Toxin status | GDH | GDH positivity rate (%) | |
|---|---|---|---|---|
| + | − | |||
| 1 CFU | Toxin+ | 18 | 24 | 42.9 |
| Toxin− | 12 | 26 | 31.6 | |
| 2 CFU ~ few | Toxin+ | 161 | 105 | 60.5 |
| Toxin− | 86 | 79 | 52.1 | |
| Some, many | Toxin+ | 566 | 38 | 93.7 |
| Toxin− | 76 | 6 | 92.7 | |
Toxin+: Toxin B PCR positive or toxigenic C. difficile isolate; Toxin−: Toxin B PCR negative or non-toxigenic isolate.
Abbreviation: GDH, glutamate dehydrogenase; CFU, colony-forming unit; PCR, polymerase chain reaction.
Based on 6,119 cases in which GDH/toxin EIA and toxin B PCR were performed, the application of guideline-based diagnostic algorithms is shown in Fig. 2.
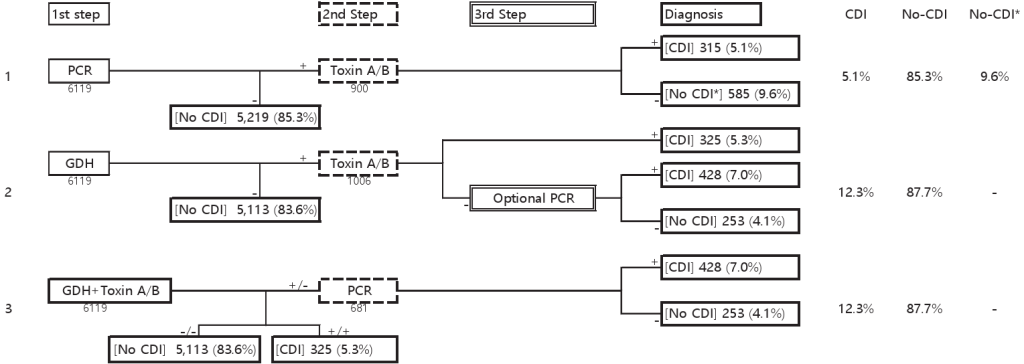
Fig. 2. Results of five years of testing applied according to the diagnostic guidelines. Abbreviations: GDH, glutamate dehydrogenase; CDI, C. difficile infection. Footnotes: CDI; CDI is likely to be present, No CDI; No further testing required. CDI is unlikely to be present, No CDI*: Clinical evaluation required (CDI or carriage of toxigenic C. difficile is possible) [2].
In Testing Algorithm 1, owing to the low sensitivity of toxin EIA, 9.6% of cases required clinical consideration of CDI or carriage of toxigenic C. difficile.
Testing Algorithm 2 required an additional step compared to Testing Algorithm 3 when optional PCR testing was performed. If PCR was omitted, 11.1% of cases, similar to Testing Algorithm 1, required clinical correlation owing to the low sensitivity of the toxin EIA.
Among the three, Testing Algorithm 3 demonstrated the highest diagnostic yield (88.9%) based solely on first-step tests without the need for further testing.
Regarding cost, Testing Algorithm 1 was the most expensive because of the extensive use of NAAT, whereas Testing Algorithm 2 was the most cost-effective.
This study evaluated the diagnostic performance of the GDH/toxin EIA test compared to toxigenic culture and C. difficile toxin B PCR over a five-year period and assessed its applicability as an initial screening tool in a two-step testing algorithm. Our findings provide important insights into the strengths and limitations of currently recommended diagnostic approaches for CDI.
GDH, a metabolic enzyme expressed in both toxigenic and non-toxigenic strains of C. difficile [9], demonstrated high NPVs of 95.1% compared to culture and 96.9% compared to toxin B PCR. These results support its potential role as a first-step screening assay. Although the sensitivities were 77.0% and 82.6%, respectively, analysis of 279 GDH(−)/culture(+) cases revealed that over 80% of the isolates exhibited minimal growth. This suggests that bacterial burden significantly affects test sensitivity and may contribute to discordant results, depending on the assay used. Table 5 further supports this finding, showing a correlation between colony growth and GDH positivity.
Toxin EIA demonstrated high specificity (99.8%) and PPV (96.9%) but low sensitivity (35.0%) compared to toxin B PCR. This is consistent with or lower than that of previous reports showing positivity rates of 36.4% and 57.9% [10,11]. Among the 585 toxin(−), PCR(+) cases, approximately half exhibited no growth or minimal growth of toxigenic strains, suggesting potential overestimation of false negatives. Table 4 reveals genotype-specific variations, with ribotype R014/020 showing a low toxin EIA positivity rate of 30.8%. This may explain the lower overall sensitivity compared to settings where high toxin-producing strains such as R027 are more prevalent. However, a significant proportion of cases demonstrated sufficient growth of toxigenic isolates despite negative toxin EIA results, raising concerns about under-diagnosis if toxin EIA is used alone.
Compared to PCR, the GDH/toxin test exhibited higher sensitivity (82.6%). All GDH-negative results were toxin EIA-negative, and all toxin EIA-positive results were GDH-positive, indicating similar performance between GDH and GDH+ toxins when using PCR as a reference. Among 157 GDH(−), toxin(−)/PCR(+) cases, culture confirmed no growth or only non-toxigenic isolates in 43 cases, suggesting potential PCR false positives. In more than half of the cases, only minimal growth of toxigenic isolates was observed.
Overall, 7.2% (1,365/18,879) of cases showed discordant results, many of which were associated with a low bacterial burden. Our institution does not apply strict criteria for CDI test requests; therefore, some cases may reflect colonization rather than true infection. Despite recommendations from major guidelines, strict two-step testing algorithms are rarely implemented in practice. Most tests are ordered simultaneously for convenience and faster turnaround time, which may reduce the cost-effectiveness of stepwise testing and complicate the clinical interpretation of discordant results.
Our findings confirmed the high NPV of the GDH and GDH/toxin tests and the high PPV of the toxin test, supporting their use in diagnostic algorithms. Assuming the implementation of three different testing algorithms at our institution, we propose that in Algorithm 1, the high cost and potential false positives of NAAT present clear disadvantages. In Algorithm 2, the addition of optional NAAT introduces complexity, whereas in Algorithm 3, using the GDH/toxin test as the first-line test offers advantages in cost, convenience, and turnaround time (Fig. 2).
The membrane-based GDH/toxin test used at our institution has demonstrated lower sensitivity compared to well-type EIAs [10,11]. Although the current PCR positivity rate at our hospital is < 15%, increasing rates may reduce the NPV, necessitating the adoption of more sensitive assays [12]. The manual nature of the test and potential for clerical errors highlight the need for workflow improvements in high-throughput settings.
Between March 2020 and January 2023, the prevalence of toxin A-negative strains (A−B+, CDT–) was less than 5%, while strains positive for toxins A, B, and binary toxins (A+B+, CDT+) accounted for less than 10% of cases. These figures are slightly higher than previously reported [13]. The most prevalent genotype was R014/020, which is consistent with the 2021 Kor-GLASS (Korean-Global Antimicrobial Resistance Surveillance System in Korea) report [14]. Although GDH positivity did not exhibit significant variation among ribotypes, toxin positivity differed across strains, suggesting potential disparities in toxin expression levels or detection sensitivity among the genotypes.
This study had several limitations. First, the clinical outcome data were not fully evaluated, limiting the ability to correlate laboratory findings with confirmed CDI cases. Second, differences in specimen quality, collection timing, and pre-analytic handling may have influenced test performance. Finally, our study was conducted at a single institution, and the findings may not be applicable to other healthcare settings with different diagnostic workflows.
The two-step testing algorithm for CDI diagnosis revealed that the GDH/toxin test demonstrated an NPV of 96.9% compared to C. difficile toxin B PCR. This suggests that the GDH/toxin test can be effectively used as a first-step test. The findings of this study support the use of either the GDH test or the GDH/toxin test as a first-line test in diagnostic stewardship for CDI.
The Institutional Review Board (IRB) of Yonsei University College of Medicine, Yongin Severance Hospital, waived the requirement for IRB review and informed consent from patients for publication (9-2021-0097).
No potential conflicts of interest relevant to this article were reported.
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-20200064).
The data sets generated in this study are available from the corresponding author upon request.
1. Wilcox MH, Planche T, Fang FC, Gilligan P. Point-counterpoint: what is the current role of algorithmic approaches for diagnosis of Clostridium difficile infection? J Clin Microbiol 2010;48:4347-53.


2. Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016;22:S63-81.

3. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:987-94.

4. Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021;116:1124-47.

5. Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, Lemeland JF, et al. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin A), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol 2004;42:5710-4.


6. Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, et al. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol 1998;36:2178-82.


7. Stubbs S, Rupnik M, Gibert M, Brazier J, Duerden B, Popoff M. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol Lett 2000;186:307-12.

8. O’Neill GL, Ogunsola FT, Brazier JS, Duerden BI. Modification of a PCR ribotyping method for application as a routine typing scheme for Clostridium difficile. Anaerobe 1996;2:205-9.
9. Carman RJ, Wickham KN, Chen L, Lawrence AM, Boone JH, Wilkins TD, et al. Glutamate dehydrogenase is highly conserved among Clostridium difficile ribotypes. J Clin Microbiol 2012;50:1425-6.


10. Lee Y, Kim M, Kim H, Lee K. Comparison of sensitivity of enzyme immunoassays for toxin A and B in different Clostridium difficile PCR ribotypes. Ann Clin Lab Sci 2014;44:38-41.
11. Chung HS, Park JS, Shin BM. Laboratory diagnostic methods for Clostridioides difficile infection: the first systematic review and meta-analysis in Korea. Ann Lab Med 2021;41:171-80.


12. Planche T, Aghaizu A, Holliman R, Riley P, Poloniecki J, Breathnach A, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis 2008;8:777-84.

13. Byun JH, Kim H, Kim JL, Kim D, Jeong SH, Shin JH, et al. A nationwide study of molecular epidemiology and antimicrobial susceptibility of Clostridioides difficile in South Korea. Anaerobe 2019;60:102106.

14. Korea Disease Control and Prevention Agency. National antimicrobial resistance surveillance in Korea 2021 annual report. https://www.kdca.go.kr/board/board.es?mid=a20310030000&bid=0132&act=view&list_no=726439&tag=&nPage=1 [Online] (last visited on 15 May 2025).
1. Wilcox MH, Planche T, Fang FC, Gilligan P. Point-counterpoint: what is the current role of algorithmic approaches for diagnosis of Clostridium difficile infection? J Clin Microbiol 2010;48:4347-53.


2. Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016;22:S63-81.

3. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:987-94.

4. Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021;116:1124-47.

5. Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, Lemeland JF, et al. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin A), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol 2004;42:5710-4.


6. Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, et al. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol 1998;36:2178-82.


7. Stubbs S, Rupnik M, Gibert M, Brazier J, Duerden B, Popoff M. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol Lett 2000;186:307-12.

8. O’Neill GL, Ogunsola FT, Brazier JS, Duerden BI. Modification of a PCR ribotyping method for application as a routine typing scheme for Clostridium difficile. Anaerobe 1996;2:205-9.
9. Carman RJ, Wickham KN, Chen L, Lawrence AM, Boone JH, Wilkins TD, et al. Glutamate dehydrogenase is highly conserved among Clostridium difficile ribotypes. J Clin Microbiol 2012;50:1425-6.


10. Lee Y, Kim M, Kim H, Lee K. Comparison of sensitivity of enzyme immunoassays for toxin A and B in different Clostridium difficile PCR ribotypes. Ann Clin Lab Sci 2014;44:38-41.
11. Chung HS, Park JS, Shin BM. Laboratory diagnostic methods for Clostridioides difficile infection: the first systematic review and meta-analysis in Korea. Ann Lab Med 2021;41:171-80.


12. Planche T, Aghaizu A, Holliman R, Riley P, Poloniecki J, Breathnach A, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis 2008;8:777-84.

13. Byun JH, Kim H, Kim JL, Kim D, Jeong SH, Shin JH, et al. A nationwide study of molecular epidemiology and antimicrobial susceptibility of Clostridioides difficile in South Korea. Anaerobe 2019;60:102106.

14. Korea Disease Control and Prevention Agency. National antimicrobial resistance surveillance in Korea 2021 annual report. https://www.kdca.go.kr/board/board.es?mid=a20310030000&bid=0132&act=view&list_no=726439&tag=&nPage=1 [Online] (last visited on 15 May 2025).