1Department of Laboratory Medicine, 2Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, 3Research Institute of Bacterial Resistance and 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
Corresponding to Young Ah Kim, E-mail: yakim@nhimc.or.kr
Ann Clin Microbiol 2021;24(1):11-20. https://doi.org/10.5145/ACM.2021.24.1.2
Received on 4 October 2020, Revised on 30 October 2020, Accepted on 3 November 2020, Published on 20 March 2021.
Copyright © Korean Society of Clinical Microbiology.
Background: The purpose of this study was to analyze the clinical and microbiological characteristics of recurrent urinary tract infection (UTI) caused by Escherichia coli— the most common etiological agent.
Methods: Cases of recurrent and single episodes of UTI caused by E. coli were evaluated retrospectively for a period of 6 months (January-June 2019) to analyze the clinical and molecular characteristics of this disease.
Results: Healthcare-associated UTI, E. coli bacteremia, and poor microbial clearance 7 days post infection were associated more with the recurrent episodes of infection. E. coli isolates from subjects with recurrent UTIs showed higher rates of antimicrobial resistance and extended-spectrum β-lactamase (ESBL) production. The E. coli clone— sequence type 131 was detected in similar proportions in isolates, recovered from subjects in both groups— recurrent episodes and single episode of UTI.
Conclusion: The control of antimicrobial-resistant ESBL-producing E. coli strains may be difficult using antimicrobial therapy and subsequently delay the clearance of the etiologic agent. This could play a major role in the development of recurrent UTIs.
Escherichia coli, Extended-spectrum β-lactamases, Recurrent urinary tract infection, Risk factors, Sequence type 131
Urinary tract infections (UTIs), which are defined microbiologically as the inflammatory response of the urothelium to microbial pathogens, are common bacterial infections. Approximately half of all women will experience symptomatic acute bacterial cystitis in their lifetime [1]. When the first infection is caused by Escherichia coli, women appear to be more likely to develop a second cystitis episode within six months than those with a first infection due to another organism [2]. In a Finnish study of women ages 17 to 82 who had E. coli cystitis, 44% had a recurrence within one year [3]. Repeated UTIs degrade the quality of life and can be accompanied by mood disturbances, such as anxiety and depression. Data on the current prevalence and factors influencing recurrent UTIs are scarce in Korea. The purpose of this study was to analyze the clinical and microbiological characteristics of recurrent UTIs by E. coli to identify the risk factors. The findings could help establish a management strategy to reduce recurrent UTIs and improve patient prognosis.
The study population was selected from non-duplicated patients admitted to the National Health Insurance Service Ilsan Hospital between January and June in 2019 and diagnosed with E. coli urinary tract infections based on culture results (n = 1,060). Patients with E. coli isolates from urine culture with 104 colony forming units (CFU)/mL or higher were included in this study. If two species were isolated from the urine culture (polymicrobial), we included only patients with E. coli at 105 CFU/mL or higher. We excluded cases with more than three species in the urine culture. For the risk factor analysis, we randomly selected cases (n=64) and controls (n=124) in which there were 213 recurrent episodes (cases) and 847 single episodes (controls). Molecular analysis was performed in 62 randomly selected E. coli isolates from subjects with recurrent episodes (two isolates were excluded because of repeated failure of cultures) and 62 E. coli isolates with single episodes,
Recurrent UTIs were defined when E. coli was isolated from urine cultures more than twice in six months. Healthcare-associated UTI was defined if the patient was transferred from a long-term care facility or diagnosed with an E. coli UTI after 48 hours of hospital admission [4]. Community-associated UTI was defined if E. coli was isolated within 48 hours of admission. The disease severity was classified by the Charlson comorbidity index [5]. Urinary tract anomalies included obstruction, hydronephrosis, renal tract calculi, and colovesical fistulas. Immunosuppressive therapy was defined as treatment with prednisolone or an equivalent drug at a dosage of at least 10 mg/day for 15 days, and chemotherapy or radiotherapy within six months before bacteremia [6]. Microbiological clearance was determined based on the results of followup urine cultures. Adequate empirical antimicrobial therapy was the administration of at least one dose of susceptible antibiotics within one calendar day of culture collection. Adequate definite antimicrobial therapy was the administration of at least one dose of susceptible antibiotics within one calendar day of culture results or within 72 hours of culture collection.
Urine cultures were performed by inoculation of clean midstream urine on blood agar and MacConkey agar and incubation at 37°C for two days. Species identification was made with a MALDI Biotyper MALDI-TOF MS (Bruker Daltonik, Bremen, Germany). Antimicrobial susceptibility testing was conducted using a MicroScan WalkAway Plus system (Beckman Coulter, Inc., West Sacramento, CA, USA) and a MicroScan Neg Breakpoint Combo Type 44 panel (Siemens Healthcare Diagnostics, Inc., West Sacramento, CA, USA) according to Clinical and Laboratory Standards Institute guidelines [7].
PCR (polymerase chain reaction) of serogroup O25b and O16 was done to determine the sequence type (ST) 131 [4]. The ESBL genotype was verified with PCR-sequencing of CTX-M, SHV, and TEMtype genes [6]. For a carbapenem-non-susceptible isolate, carbapenemase genes were identified by PCR and sequenced using primers to detect IMP, KPC, NDM, VIM, and OXA-48 [8]. Pulsed-field gel electrophoresis (PFGE) was performed as described in our previous study [6]. A total of 124 isolates were included from recurrent episodes (n = 62) and single episodes of UTIs (n = 62). A total of 122 E. coli isolates were finally analyzed by PFGE because of repeated fails in two E. coli isolates. The patterns were analyzed using InfoQuest FP software (Bio-Rad, Hercules, CA, USA) to generate a dendrogram based on the unweighted pair group method, with an arithmetic average (UPGMA) from the Dice coefficient with 1% band position tolerance and 0.5% optimization settings.
The first episode was included in the analysis. Clinical data were retrospectively obtained from a review of the electronic medical record. The included variables were age, sex, underlying disease, Charlson comorbidity index (CCI), urinary tract anomaly, acquisition site, transfer from another healthcare facility, appropriate antimicrobial treatment (empirical and final), microbiological clearance, 30-day mortality, and ESBL E. coli phenotype at the first episode. Infection evidence, such as fever, laboratory findings (C-reactive protein, albumin, white blood cell count), and blood cultures were also reviewed. Up to 90 days from the first episode, previous surgery, use of Foley catheter, immunosuppressive therapy, and total parenteral nutrition, and admission to an intensive care unit were recorded.
Continuous variables, such as age, were analyzed by the Mann-Whitney U test. The chi-squared test was used for comparative analysis of the categorical variables to determine the independent risk factors. Odds ratio (OR) and 95% confidence interval (CI) values were calculated for the binomial variables. Variables with P-values less than 0.1 in univariate analyses were included in a multivariate logistic regression analysis model to determine the independent risk factors. Statistical significance was defined as P < 0.05. SPSS 23.0 software (SPSS, Chicago, IL, USA) was used for the univariate and multivariate analyses. This study was approved by the Institutional Review Boards as required by the hospital policy (IRB No. NHIMC 2020-03002).
The E. coli isolates from patients with recurrent UTIs were much less sensitive to antimicrobial agents than those isolated from patients with single episodes, except for piperacillin-tazobactam, amoxicillinclavulanic acid, cefoxitin, and minocycline (Fig. 1). Only one E. coli isolate was resistant to doripenem, meropenem, and imipenem, which was from a subject with recurrent UTIs. Neither colistin nor tigecyclineresistant E. coli were isolated from patients in either group.
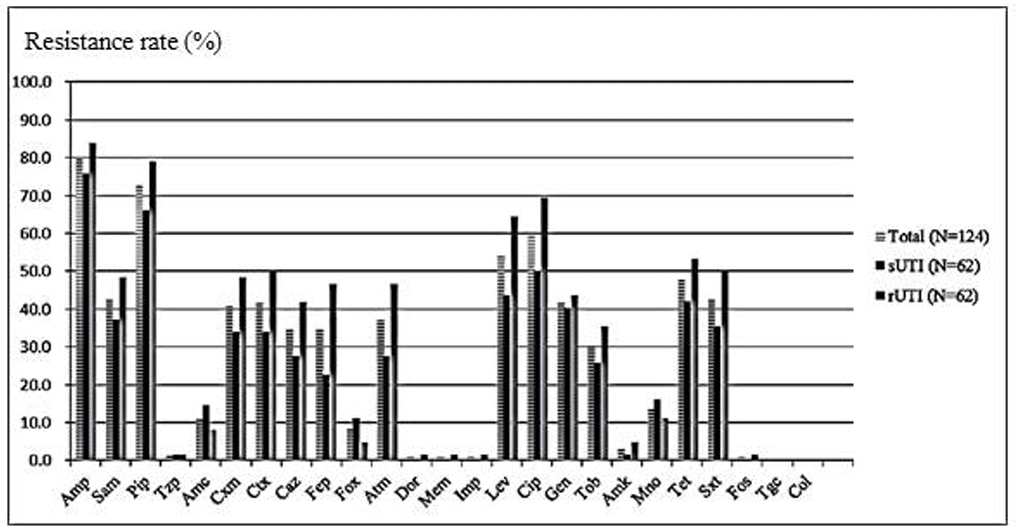
Fig. 1. Antimicrobial resistance rates of Escherichia coli. Total UTI (n = 124) included both recurrent episodes (n = 62) and single episodes (n = 62).
Abbreviations: rUTI, recurrent urinary tract infection; sUTI, single episode of urinary tract infection; N, number, Amp, ampicillin; Sam, ampicillin-sulbactam; Pip, piperacillin; Tzp, piperacillin-tazobactam; Amc, amoxicillin-clavulanic acid; Cxm, cefuroxime; Ctx, cefotaxime; Caz, ceftazidime; Fep, cefepime; Fox, cefoxitin; Atm, aztreonam; Dor, doripenem; Mem, meropenem; Imp, imipenem; Lev, levofloxacin; Cip, ciprofloxacin; Gen, gentamicin; Tob, tobramycin; Amk, amikacin; Mno, minocycline; Tet, tetracycline; Sxt, trimethoprim-sulfamethoxazole; Fos, fosfomycin; Tgc, tigecycline; Col, colistin.
The ST 131 E. coli clone was frequently detected but the proportions were similar in both groups (35.5% in recurrent episodes and 30.7% in single episodes). The rate of ESBL producers in the recurrent episode group was higher than that of the single episode group (45.2% versus 21.0%, P = 0.0042) but genotypes CTX-M-15 and CTX-M-14 were common, i.e., more than 20% in both groups (Table 1). KPC2 carbapenemase was detected in a carbapenem-resistant E. coli. There was no dominant clone in the PFGE patterns using an 80% cut-off (Fig. 2).
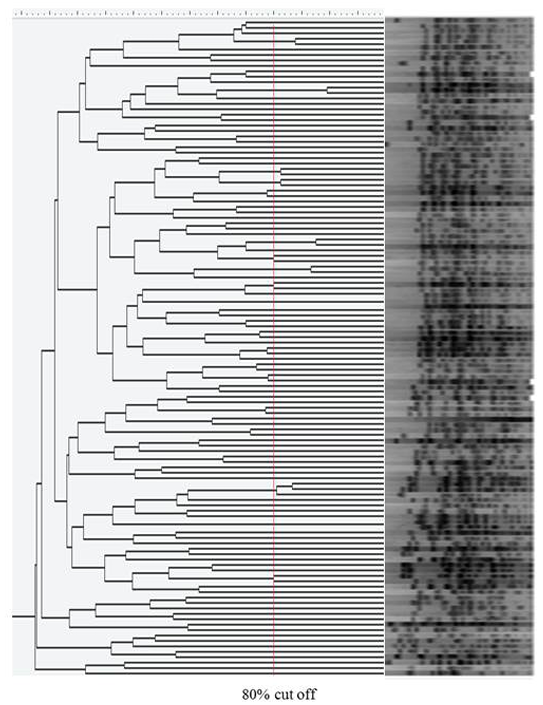
Fig. 2. Dendrogram of XbaI-restricted DNA of Escherichia coli isolated from urinary tract infection (n=122). PFGE was performed with size marker, Lambda Ladders (Promega, Fitchburg, WI, USA).
Abbreviations: ESBL, extended-spectrum β-lactamase; ST, sequence type; rUTI, recurrent urinary tract infection; sUTI, single episode of E. coli urinary tract infection.
Table 1. The molecular epidemiology of E. coli isolated from urinary tract infections
| Characteristic | No. (%) in Recurrent episodes | No. (%) in Single episodes | P-value |
|---|---|---|---|
| ST131 | 22/62 (35.5) | 19/62 (30.6) | 0.5669 |
| O25 | 20/22 (90.9) | 19/19 (100) | |
| O16 | 02/22 (9.1) | 00/19 (0) | |
| ESBL genotype | 28/62 (45.2) | 13/62 (30.8) | 0.0042* |
| CTX-M-14-type | 06/28 (21.4) | 04/13 (46.2) | 0.0619† |
| CTX-M-15-type | 15/28 (53.6) | 06/13 (30.0) | |
| CTX-M-27-type | 03/28 (10.7) | 02/13 (15.4) | |
| CTX-M-55-type | 04/28 (14.4) | 01/13 (7.7) |
The molecular analysis was performed in 62 cases and 62 controls at a 1:1 ratio. *The difference of ESBL production rates in both groups. †The difference of ESBL genotypes in both groups. Abbreviations: ST, sequence type; ESBL, extended-spectrum β-lactamase.
When the two groups of patients with E. coli UTIs were compared, hemiplegia (12.5% in recurrent episodes versus 2.4% in single episodes, P = 0.0053), healthcare-associated acquisition (21.9% in recurrent episodes versus 5.7% in single episodes, P = 0.0008), and the isolation of E. coli in follow-up urine cultures after seven days (28.1% in recurrent episodes versus 1.6% in single episodes, P < 0.0001) were found more in patients with recurrent episodes than in single episodes (Table 2). However, adequate antimicrobial treatment was more common in patients with single episodes, regardless of empirical treatment (34.4% in recurrent episodes versus 56.5% in single episodes, P = 0.0041) or definite treatment (45.3% in recurrent episodes versus 64.5% in single episodes, P = 0.0115).
Table 2. Comparison of Recurrent and single episodes of urinary tract infections caused by E. coli
| Variable | No. (%) in Recurrent episodes (n = 64) | No. (%) in Single episodes (n = 124) | P-value |
|---|---|---|---|
| Age in years | 71.8 ± 18.8 | 71.6 ± 16.8 | 0.9362 |
| Male sex | 15 (23.4) | 17 (13.7) | 0.0926 |
| Acquisition site | |||
| Community-associated* | 50 (78.1) | 117 (94.4) | 0.0008 |
| Healthcare-associated | 14 (21.9) | 07 (5.7) | |
| Transfer from | |||
| Long-term care facility | 02 (3.1) | 04 (3.2) | 0.9618 |
| Acute care facility | 16 (25.0) | 27 (21.8) | |
| Clinics | 16 (25.0) | 34 (27.4) | |
| Associated disease | |||
| Diabetes mellitus | 24 (37.5) | 35 (28.2) | 0.1941 |
| Chronic renal disease | 11 (17.2) | 16 (12.9) | 0.4274 |
| Liver cirrhosis | 01 (1.6) | 02 (1.6) | 0.9792 |
| Kidney transplantation | 01 (1.6) | 01 (0.8) | 0.6321 |
| Cancer | 06 (9.4) | 16 (12.9) | 0.4758 |
| BPH (male only) | 04 (26.7) | 05 (29.4) | 0.8632 |
| Hemiplegia | 08 (12.5) | 03 (2.4) | 0.0053 |
| Cerebrovascular disease | 08 (12.5) | 18 (14.5) | 0.7044 |
| Dementia | 04 (6.3) | 15 (12.1) | 0.2076 |
| Charlson comorbidity index | 2.6 ± 1.5 | 2.5 ± 1.9 | 0.6923 |
| Urinary tract anomaly | 05 (7.8) | 07 (5.7) | 0.5646 |
| Surgical history | 02 (3.1) | 06 (4.8) | 0.5812 |
| Urinary catheter use | 38 (59.4) | 70 (56.5) | 0.7009 |
| Immunosuppressive therapy | 01 (1.6) | 01 (0.8) | 0.6321 |
| TPN use | 07 (10.9) | 16 (12.9) | 0.6967 |
| ICU admission | 01 (1.6) | 09 (7.3) | 0.0992 |
| Fever (> 37.5°C) | 08 (12.5) | 17 (13.7) | 0.8170 |
| Laboratory findings | |||
| C-reactive protein (mg/dL, n=132) | 6.8 ± 8.8 | 8.9 ± 8.3 | 0.1182 |
| WBC (10³/μL, n=152) | 12.0 ± 7.5 | 10.9 ± 6.1 | 0.3580 |
| Albumin (g/dL, n=128) | 3.4 ± 0.7 | 3.4 ± 0.7 | 0.3657 |
| Microbiological clearance | |||
| Clearance within 7 days | 19 (29.7) | 35 (28.2) | <0.0001 |
| Persistence after 7 days | 18 (28.1) | 02 (1.6) | |
| E. coli bacteremia | 17 (26.6) | 19 (15.3) | 0.0635 |
| Adequate empirical antibiotic use | 22 (34.4) | 70 (56.5) | 0.0041 |
| Adequate definite antibiotic use | 29 (45.3) | 80 (64.5) | 0.0115 |
Data are numbers (%), except for age, Charlson comorbidity index, and laboratory findings, which are mean ± standard deviation.
*Bold formatting indicates statistical significance. Abbreviations: rUTI, recurrent urinary tract infection; sUTI, single episode of urinary tract infection; BPH, benign prostate hyperplasia; TPN, total parenteral nutrition; ICU, intensive care unit; WBC, white blood cell.
Healthcare-associated UTIs (OR = 3.731, P = 0.0239), E. coli bacteremia (OR = 2.580, P = 0.0344), and no microbiological clearance after seven days (OR = 13.792, P =0.0020) were more associated with recurrent episodes in multivariate analysis (Table 3).
Table 3. Risk factors for recurrent urinary tract infections by E. coli by multivariate analysis
| Risk factor* | OR (95% CI) | P-value |
|---|---|---|
| Healthcare-associated | 03.731 (1.190-11.698) | 0.0239 |
| E. coli bacteremia | 02.580 (1.072-6.209) | 0.0344 |
| Persistence after 7 days | 13.792 (2.603-73.063) | 0.0020 |
*Statistical significances were maintained after the adjustment for sex, underlying disease (hemiplegia), acquisition sites, intensive care unit admission, E. coli bacteremia, microbiological clearance, and adequate antibiotic treatment (empirical and definite). Abbreviations: OR, odds ratio; CI, confidence interval.
E. coli is the most common etiology of UTIs and 6,394 (11.1%) E. coli isolates were recovered from 57,477 urine cultures according to the first one-year report from Korea Global Antimicrobial Resistance Surveillance System (2016-2017) [4]. For the treatment of UTI, oral fluoroquinolone and oral cephalosporin are usually recommended for non-complicated cystitis and ceftriaxone and fluoroquinolone are strongly recommended according to the results of susceptibility testing for adult simple acute pyelonephritis [9]. However, the resistance rates in the E. coli urine isolates to cefotaxime, ceftazidime, and ciprofloxacin were high [4] because extended-spectrum-β-lactamase-producing E. coli (ESBL-EC) has increased in both community and hospital settings in Korea [10,11]. ESBL-EC breaks down extended-spectrum cephalosporin and aztreonam, showing wide-ranging resistance to cephalosporins except for carbapenem. Specifically, ST 131 E. coli, a high-risk clone with extensive antibacterial resistances, high-virulent traits, and increased propagation power, has contributed to the worldwide dissemination. ESBL-EC had multidrug
resistance traits, which shows resistance to other classes, such as fluoroquinolone and aminoglycoside [12].
Old age, female, catheterization, and sexual activity are well-known risk factors of UTI [13] but the molecular epidemiology and risk factors of recurrent UTI have not been fully evaluated in Korea. Both bacterial factors and deficiencies in host defense could contribute to the pathogenesis of recurrent UTIs [14]. Bacterial survival in the urinary bladder after antibiotic treatment and progression to forming intracellular bacterial communities might be the most important bacterial factors. Immunodeficiency and urogenital tract anatomical abnormalities have been considered essential risk factors for recurrent UTIs.
In this study, the rates of ESBL production and antimicrobial resistance were different between the two groups of patients with recurrent episodes and single episodes. The differences in microbial clearance and the proportions of adequate antimicrobial treatment were also noticeable in the two groups. However, the proportions of associated disease, disease severity, urinary tract anomalies, and immunosuppressive therapy were similar between the two groups. We inferred that the multidrug-resistant traits of ESBL-EC may hinder adequate antimicrobial therapy and consequently delay the clearance of the etiologic agents, which could play a major part in the development of recurrent UTIs. Risk factors, such as healthcare-associated UTIs, E. coli bacteremia, and no microbiological clearance after seven days, were more associated with recurrent episodes, which could be used as predictors.
The catheter-associated UITs were more associated with older age, diabetes mellitus, chronic renal disease, cancer, cerebrovacular accidents, dementia, higher CCI, surgical history, total parenteral nutrition use, intensive care unit admission, concommittant E. coli bacteremia, higher 30 day mortality and more ESBL phenotypes (data was not shown).
This study could provide recent data, including microbiology and molecular features, in addition to clinical features and antimicrobial treatments. The limitation of this study was that we defined UTIs based on urine culture results without consideration of UTI clinical features. As a result, asymptomatic bacteriuria could be included, even though we set the criteria to rule out contaminated urine cultures. Another limitation of this study, the used breakpoints are intended for the bloodstream infection. Because the definition of adequate antimicrobial therapy was based on this result, the resistance to specific antimicrobials did not definitely indicate UTI treatment failure due to a high concentration of antimicrobials in the urine. These two limitations possibly affected the low rates of empirical and definitive antimicrobial treatment.
In this study, we could not discriminate between reinfections or relapses in the recurrent episodes. Most recurrences are thought to represent reinfections rather than relapses (even recurrences caused by the same uropathogenic strain), although occasionally, a persistent focus can produce a relapsing infection [15]. However, others have said that it is because phenotypically based typing methods or less specific DNAbased typing methods were used [15]. It was reported that most recurrent UTIs were caused by relapse with the primary infecting E. coli according to PFGE analysis and may support the recent observation that E. coli could be an intracellular reservoir for recurrent UTIs [16]. Further studies are needed to evaluate the microbiological nature of recurrent episodes.
배경: 본 연구의 목적은 요로감염의 가장 흔한 원인균인 Escherichia coli에 의한 반복적 요로감염의 임상 및 미생물학적 특성을 분석하여 위험인자를 파악하는 것이다.
방법: 2019년 1월부터 6개월동안 E. coli에 의한 반복적 요로감염 환자와 단일 에피소드를 후향적으로 비교하였다.
결과: 반복적 요로감염 환자에서 의료기관 관련 요로감염, 대장균 균혈증 동반, 소변 추적배양에서 음전되지 않은 경우가 많았다. 반복적 요로감염 환자에서 분리된 E. coli는 extended-spectrum β-lactamase (ESBL) 생성이 빈번하고 항균제 내성률이 높았다. 자주 검출되는 ST131 E. coli의 비율은 두 군이 유사하였다.
결론: ESBL을 생성하는 E. coli는 흔히 다제내성을 보이므로 적절한 항균제 치료의 가능성을 낮추어 요로 내 E. coli의 제거를 지연시켜 반복적인 요로감염과 관련성이 큰 것으로 생각된다.
No potential conflicts of interest relevant to this article were reported.
I would like to thank So Ra Yoon, PhD for the statistics from research institute of national health insurance ilsan hospital.
1. Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: a population-based assessment. Infection 2007;35:150-3.

2. Jhang JF, Kuo HC. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med J 2017;29:131-7.


3. Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallma P, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol 2000;151:1194-205.

4. Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017; first one-year report from KorGLASS. Euro Surveill 2018;23.

5. Robbins JR, Gayar OH, Zaki M, Mahan M, Buekers T, Elshaikh MA. Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer. Gynecol Oncol 2013;131:593-7.

6. Kim H, Kim YA, Park YS, Choi MH, Lee GI, Lee K. Risk factors and molecular features of sequence type (ST) 131 extended-spectrum β-lactamase-producing Escherichia coli in community-onset bacteremia. Sci Rep 2017;7:14640.


7. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 25th informational supplement. Wayne; PA: 2015.
8. Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011;17:1791.


9. Kang CI, Kim J, Park DW, Kim BN, Ha U, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother 2018;50:67-100.


10. Kim YA, Kim JJ, Kim H, Lee K. Community-onset extended-spectrum-β-lactamase-producing Escherichia coli sequence type 131 at two Korean community hospitals: the spread of multidrug-resistant E. coli to the community via healthcare facilities. Int J Infect Dis 2017;54:39-42.

11. Kim S, Park Y, Johnson JR, Yu JK, Kim Y, Kim YS. Prevalence and characteristics of Escherichia coli sequence type 131 and its H30 and H30Rx subclones: a multicenter study from Korea. Diagn Microbiol Infect Dis 2016;84:97-101.

12. Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 2011;66:1-14.

13. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019;11:3-7.


14. Jhang JF, Kuo HC. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med J 2017;29:131-7.


15. Ejrnæs K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull 2011;58:B4187.
16. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 2007;4:e329.


1. Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: a population-based assessment. Infection 2007;35:150–3.
2. Jhang JF, Kuo HC. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med J 2017;29:131-7.
3. Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallma P, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol 2000;151:1194-205.
4. Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017; first one-year report from KorGLASS. Euro Surveill 2018;23.
5. Robbins JR, Gayar OH, Zaki M, Mahan M, Buekers T, Elshaikh MA. Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer. Gynecol Oncol 2013;131:593-7.
6. Kim H, Kim YA, Park YS, Choi MH, Lee GI, Lee K. Risk factors and molecular features of sequence type (ST) 131 extended-spectrum β-lactamase-producing Escherichia coli in community-onset bacteremia. Sci Rep 2017;7:14640.
7. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 25th informational supplement. Wayne; PA: 2015.
8. Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011;17:1791.
9. Kang CI, Kim J, Park DW, Kim BN, Ha U, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother 2018;50:67-100.
10. Kim YA, Kim JJ, Kim H, Lee K. Community-onset extended-spectrum-β-lactamaseproducing Escherichia coli sequence type 131 at two Korean community hospitals: the spread of multidrug-resistant E. coli to the community via healthcare facilities. Int J Infect Dis 2017;54:39-42.
11. Kim S, Park Y, Johnson JR, Yu JK, Kim Y, Kim YS. Prevalence and characteristics of Escherichia coli sequence type 131 and its H30 and H30Rx subclones: a multicenter study from Korea. Diagn Microbiol Infect Dis 2016;84:97–101.
12. Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 2011;66:1–14.
13. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019;11:3-7.
14. Jhang JF, Kuo HC. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med J 2017;29:131-7.
15. Ejrnæs K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull 2011;58:B4187.
16. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 2007;4:e329.