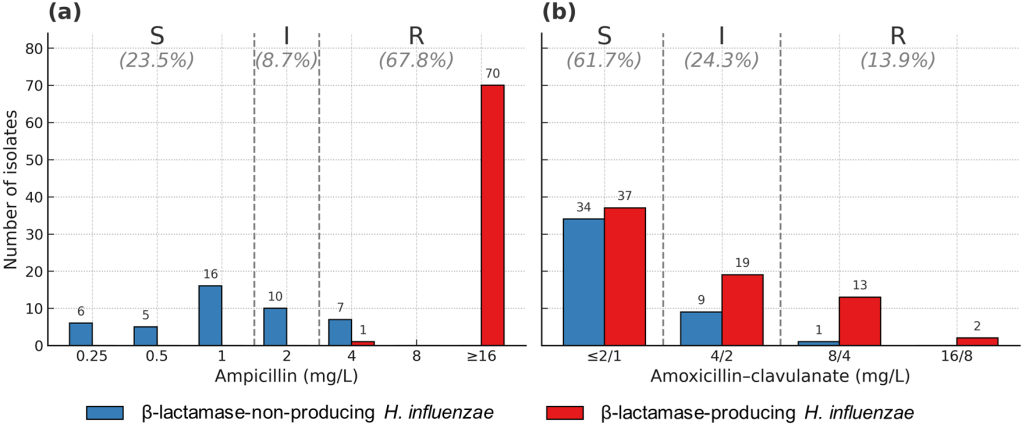
Fig. 1. Antimicrobial susceptibility of Haemophilus influenzae isolates to ampicillin (a) and amoxicillin–clavulanate (b) in South Korea. S, susceptible; I, intermediate; R, resistant.
Ann Clin Microbiol 2025;28(4):23. Prevalence and molecular characteristics of β-lactam resistance in non-typeable Haemophilus influenzae isolates in Korea Download image
Table 2. Amoxicillin–clavulanate susceptibility of ampicillin-resistant Haemophilus influenzae isolates
Ann Clin Microbiol 2025;28(4):23. Prevalence and molecular characteristics of β-lactam resistance in non-typeable Haemophilus influenzae isolates in Korea Download table Amoxicillin–clavulanate Number of ampicillin-resistant isolates (%) β-lactamase producer (n = 71) β-lactamase non-producer (n = 7) Total (n = 78) Resistant 15 (93.8) 1 (6.2) 16 (100.0) Intermediate 19 (79.2) 5 (20.8) 24 (100.0) Susceptible […]
Table 1. Primers used in the study
Ann Clin Microbiol 2025;28(4):23. Prevalence and molecular characteristics of β-lactam resistance in non-typeable Haemophilus influenzae isolates in Korea Download table Target genes Primer name Sequences (5’→3′) Size (bp) References blaTEM TEM (321) TGGGTGCACGAGTGGGTTAC 526 [11] TEM (846) TTATCCGCCTCCATCCAGTC blaROB ROB (419) ATCAGCCACACAAGCCACCT 692 ROB (1110) GTTTGCGATTTGGTATGCGA ftsI F1 (936) GTTAATGCGTAACCGTGCAATTACC 704 [12] F2 (1640) ACCACTAATGCATAACGAGGATC […]
Table 2. Categorical agreement and error rates between the reference method and the Sensititre SLOMYCO panel
Ann Clin Microbiol 2025;28(4):24. Drug susceptibility testing of Mycobacterium avium complex using the SLOMYCO test-system: a diagnostic accuracy study Download table Antimicrobials No. of isolates tested % Categorical agreement (95% CI) Very major error (VME, %) Major error (ME, %) Minor error (mE, %) Amikacin 86 77.9 (68.0-85.4) 0 2.3 19.8 Clarithromycin 86 100.0 (95.7-100.0) […]
Table 1. Comparison of MICs and EA between the reference broth microdilution and Sensititre SLOMYCO panel for mycobacterial isolates
Ann Clin Microbiol 2025;28(4):24. Drug susceptibility testing of Mycobacterium avium complex using the SLOMYCO test-system: a diagnostic accuracy study Download table Antimicrobials Method No. of isolates with each MIC (µg/mL) No. of isolates included for the EA calculation EA (%) 95% CI of EA Amikacin MIC ≤1 1 2 4 8 16 32 64 >64 […]
Table 4. Functions of resistance-associated genes and drug resistance mechanisms in Mycobacterium tuberculosis [20]
Ann Clin Microbiol 2025;28(4):26. Drug susceptibility testing for Mycobacterium tuberculosis: a narrative review Download table WHO category Drug or drug class Resistance gene Gene function Mechanism of drug resistance First-line agents Rifampicin rpoB RNA polymerase Target modification Isoniazid katG Catalase-peroxidase enzyme Decreased drug activation inhA NADH-dependent enoyl-acyl carrier protein Target amplification or modification Pyrazinamide pncA […]
Table 3. Critical concentrations of drugs recommended for treating drug-susceptible and multidrug- or rifampicin-resistant tuberculosis
Ann Clin Microbiol 2025;28(4):26. Drug susceptibility testing for Mycobacterium tuberculosis: a narrative review Download table Category Drug Critical concentrations (μg/mL) by medium LJ Middlebrook 7H10 Middlebrook 7H11 MGIT Drug-susceptible TB First-line drugs Rifampicin 40.0 0.5 1.0 0.5 Isoniazid 0.2 0.2 0.2 0.1 Ethambutola) 2.0 5.0 7.5 5.0 Pyrazinamidea) – – – 100.0 MDR/RR-TB […]
Table 2. Classification of drugs used in multidrug- and rifampicin-resistant tuberculosis treatment regimens in Korea [5]
Ann Clin Microbiol 2025;28(4):26. Drug susceptibility testing for Mycobacterium tuberculosis: a narrative review Download table Group Anti-TB drug A Levofloxacin or MoxifloxacinBedaquilineDelamanidLinezolid B CycloserineClofazimine C Amikacin or Kanamycin (Streptomycin)EthambutolImipenem-cilastatin or MeropenemPara-aminosalicylc acid (PAS)ProthionamidePyrazinamide Group A: Highly effective core medicines that should be included in all longer MDR/RR-TB regimens unless contraindicated due to toxicity, intolerance, or […]
Table 1. Drug-resistant tuberculosis classifications
Ann Clin Microbiol 2025;28(4):26. Drug susceptibility testing for Mycobacterium tuberculosis: a narrative review Download table Drug-resistance Description Isoniazid-resistant TB (rifampicin-susceptible) (Hr-TB) Tuberculosis caused by Mycobacterium tuberculosis strains that are resistant to isoniazid but remain susceptible to rifampicin. Rifampicin-resistant TB (RR-TB) TB caused by strains resistant to rifampicin, with or without resistance to other first-line drugs. […]
Whole-genome sequencing as the new framework of clinical microbiology and highlights in this issue
Editorial Hae-Sun Chung Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea Correspondence to Hae-Sun Chung, E-mail: sunny0521.chung@ewha.ac.kr Ann Clin Microbiol 2025;28(4):27. https://doi.org/10.5145/ACM.2025.28.4.8Received on 16 December 2025, Revised on 18 December 2025, Accepted on 18 December 2025, Published on 20 December 2025.Copyright © Korean Society of Clinical Microbiology.This is an Open Access […]
