Eris Jang1![]() , So Min Kim2
, So Min Kim2![]() , Junhyup Song2
, Junhyup Song2![]() , Le Phuong Nguyen2,3
, Le Phuong Nguyen2,3![]() , Hyukmin Lee2
, Hyukmin Lee2![]()
1Sprayberry High School, Marietta, USA, 2Department of Laboratory Medicine and Research Institute of Bacterial Resistance, 3Brain Korea 21 plus Program for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Corresponding to Junhyup Song, E-mail: llive@yuhs.ac
Ann Clin Microbiol 2021;24(2):61-65. https://doi.org/10.5145/ACM.2021.24.2.4
Received on 20 April 2021, Revised on 20 April 2021, Accepted on 20 April 2021, Published on 20 June 2021.
Copyright © Korean Society of Clinical Microbiology.
Roseomonas aerofrigidensis is a gram-negative, strictly aerobic, non-motile bacterium, which was first isolated in 2017 in South Korea. We present the first report of the isolation of R. aerofrigidensis from the peritoneal fluid of a 38-year-old woman with a history of metastatic gastric cancer with peritoneal carcinomatosis. The isolate was resistant to cotrimoxazole. Further research on clinical and microbiological responses to several antibiotics are warranted.
Gastric Cancer Patient, Peritonitis, Roseomonas aerofrigidensis
Roseomonas aerofrigidensis is a gram-negative, strictly aerobic, non-motile bacterium that was first isolated in 2017 from an air conditioner in South Korea [1]. Many recent reports of Roseomonas species come from various non-clinical settings [1–3]. However, they were initially found in clinical samples, often being reported as opportunistic pathogens [4–8]. Especially, Roseomonas gilardii and R. mucosa were most frequently reported as the ones responsible for human infections [6–8]. There have not been any worldwide reports of R. aerofrigidensis to be isolated from a clinical sample before. To the best of our knowledge, we report the first case of R. aerofrigidensis to be isolated from an immunocompromised patient.
A 38-year old woman with a history of metastatic gastric cancer with peritoneal carcinomatosis was admitted to Severance Hospital due to general weakness and neutropenia in 17 April 2020. The patient was diagnosed with metastatic cancer on May 2019 and continued her treatment with a combination of oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX) on an outpatient basis since then. After changing the regimen to what consisted of leucovorin calcium, 5-fluorouracil, and irinotecan (FOLFIRI) on 3 April 2020, the absolute neutrophil count of the patient decreased to 740/μL. On the first day of hospitalization, the body temperature of the patient was sustained over 38.1°C. Laboratory findings showed decreased white blood cell count of 3.02×103/μL (24.4% segmented neutrophils) and elevated C-reactive protein (CRP) level (40.1 mg/L). Abdominal computerized tomography indicated increased amount of ascites compared to 2 months ago. The patient was diagnosed as having neutropenic fever and empirically treated with cefepime.
A sample was collected from a peritoneal fluid that was taken on the 6th day of hospitalization and obtained through an indwelling catheter. The sample was cultured on 5% sheep blood agar and MacConkey agar. After 24 hours of incubation at 35°C, grey and mucoid colonies were shown on the sheep blood agar plate, but no growth was observed MacConkey agar (Fig. 1A). For a clearer depiction, gram staining also took place, which revealed that the bacteria were gram-negative cocci (Fig. 1B). Additionally, they came out positive for both the oxidase and catalase testing. Blood culture was also performed on the same day as the peritoneal fluid drainage, and there were no bacterial isolates grown on blood culture. A follow-up culture for both the peritoneal fluid and blood sample was not performed as the patient’s condition was rapidly improved after the start of empirical antibiotic treatments. The matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Breman, Germany) with the analysis softwares, flexAnalysis 3.4 and MALDI Biotyper 3 failed to identify the isolate. The reference spectra for Roseomonas aerofrigidensis was not registered in the database. In Rosemonas genera, only Roseomonas mucosa was registered in the database. The VITEK 2 identification system (bioMérieux, Marcy l’Etoile, France) also failed to identify the isolate. Species identification was followed by the CLSI MM18-A2 guidelines [9]. DNA amplification of 16S rRNA gene was performed using the universal long primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The 1347 bp consensus sequence from sanger sequencing was compared with the BLAST database (https:// blast.ncbi.nlm.nih.gov) and the EzBiocloud database (https://www.ezbiocloud.net/identify).
There was no difference in the results of the best match and the second best match between the BLAST and the EzBiocloud database. The isolate was identified as R. aerofrigidensis (GenBank accession number KY126356) with 99.70% sequence identity. Furthermore, the sequence of the isolate shared 98.57% sequence identity with that of Roseomonas oryzae (GenBank accession number LN810637) showing 1.13% sequence divergence from R. aerofrigidensis. (Fig. 1C)
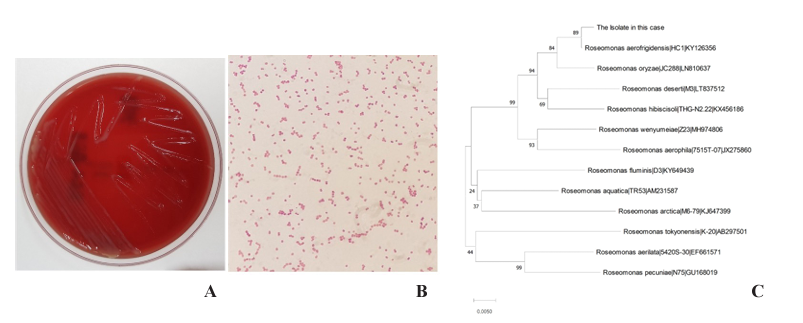
Fig. 1. Colony morphology, microscopic image and genetic relationship with related taxa of R. aerofrigidensis. (A) Colonies on sheep blood agar. (B) Gram staining of the isolate (1,000×). (C) A neighbor-joining tree based on 16S rRNA gene sequences. The scale bar equals 0.0050 changes per nucleotide position. Bootstrap values are shown on nodes as percentages of 1,000 replicates.
Antimicrobial susceptibility testing (AST) of the R. aerofrigidensis was first performed using the VITEK 2 identification system with the AST-N225 card. However, the VITEK 2 system was unsuccessful. The E-test (bioMérieux, Marcy l’Etoile, France) was later performed to determine the minimum inhibitory concentration for multifold antibiotics. The results were interpreted by using CLSI guidelines [10] and showed that the isolate was susceptible to cefotaxime and levofloxacin and resistant to cotrimoxazole (Table 1).
The patient showed a good clinical response to an empirical administer of intravenous cefepime. The patient’s condition improved within a week after antibiotic treatments. The fever subsided and the CRP went down to 3.6 mg/L. The patient was discharged after the follow-up peritoneal fluid culture, which did not show any growth of other possible pathogens.
Table 1. The results of the antimicrobial susceptibility test
| Antimicrobial agents | MIC (μg/mL) | Susceptibility |
|---|---|---|
| Cefotaxime | 1.5 | S |
| Levofloxacin | 0.125 | S |
| Cotrimoxazole | ≥32 | R |
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; R, resistant.
This is the first case of R. aerofrigidensis to be isolated from a clinical sample. The isolate could not be identified with the MALDI-TOF MS and the VITEK 2 system, but the 16S rRNA sequencing revealed that the isolate collected from the patient matched 99.70% with R. aerofrigidensis, which was only isolated once prior to this report [1]. Since there are very limited resources about R. aerofrigidensis, additional studies about its pathogenicity is required. Moreover, as the isolate showed resistance to cotrimoxazole, gathering more data for clinical and microbiological responses to several antibiotics would be warranted.
Roseomonas aerofrigidensis는 그람 음성 호기성 구균으로 2017년 한국에서 환경으로부터의 분리가 최초 보고된 균이다. 복막으로 전이된 위암 과거력을 가진 38세 여자 환자가 전신쇠약과 호중구 감소증으로 입원하였다. 입원 1일 째 환자는 38.1°C의 발열과 40.1 mg/L로 증가된 C-reactive protein (CRP) 수치를 보였다. 입원 6일 째 환자의 복수 배양을 통한 그람 염색에서 그람 음성 구균을 확인하였고, oxidase 및 caltalase 검사에서 양성 소견을 보였으며 matrix-assisted laser desorption/ ionization time-of-flight 질량분석기(MALDI-TOF MS; Bruker Daltonics, Germany) 와 VITEK 2 식별 시스템(bioMérieux, France) 에서 동정이 되지 않았으며, 분자 식별 검사 상 GenBank Basic Local Alignment Search Tool과 Ez-Taxon 데이터베이스 검색 결과 R. aerofrigidensis 와 99.70%의 상동성을 보였다. 이것은 R. aerofrigidensis에 의한 인체 감염의 최초 사례이다.
No potential conflicts of interest relevant to this article were reported.
We are grateful to all laboratory members for providing constructive criticism on this manuscript. We would like to explicitly state that Eris Jang performed most essential parts of this research including collection of the data and preparing of the tables, figures, and manuscript, during her 1 month internship in Research Institute of Bacterial Resistance of Yonsei University College of Medicine.
1. Hyeon JW and Jeon CO. Roseomonas aerofrigidensis sp. nov., isolated from an air conditioner. Int J Syst Evol Microbiol 2017;67:4039-44.

2. Kim SJ, Weon HY, Ahn JH, Hong SB, Seok SJ, Whang KS, et al. Roseomonas aerophila sp. nov., isolated from air. Int J Syst Evol Microbiol 2013;63:2334-7.

3. Furuhata K, Ishizaki N, Edagawa A, Fukuyama M. Roseomonas tokyonensis sp. nov. isolated from a biofilm sample obtained from a cooling tower in Tokyo, Japan. Biocontrol Sci 2013;18:205-9.

4. Kim YK, Moon JS, Song KE, Lee WK. Two cases of bacteremia due to Roseomonas mucosa. Ann Lab Med 2016;36:367-70.


5. Dé I, Rolston KV, Han XY. Clinical significance of Roseomonas species isolated from catheter and blood samples: analysis of 36 cases in patients with cancer. Clin Infect Dis 2004;38:1579-84.

6. Kim KY, Hur J, Jo W, Hong J, Cho OH, Kang DH, et al. Infectious spondylitis with bacteremia caused by Roseomonas mucosa in an immunocompetent patient. Infect Chemother 2015;47:194-6.


7. Bibashi E, Sofianou D, Kontopoulou K, Mitsopoulos E, Kokolina E. Peritonitis due to Roseomonas fauriae in a patient undergoing continuous ambulatory peritoneal dialysis. J Clin Microbiol 2000;38:456-7.


8. Struthers M, Wong J, Janda JM. An initial appraisal of the clinical significance of Roseomonas species associated with human infections. Clin Infect Dis 1996;23:729-33.

9. Clinical and Laboratory Standards Institute (CLSI). Interpretive criteria for identification of bacteria and fungi by targeted DNA sequencing; approved guideline MM18-ED2. Wayne; PA:2018.
10. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; approved guideline M100-ED30. Wayne; PA:2019.
1. Hyeon JW and Jeon CO. Roseomonas aerofrigidensis sp. nov., isolated from an air conditioner. Int J Syst Evol Microbiol 2017;67:4039-44.
2. Kim SJ, Weon HY, Ahn JH, Hong SB, Seok SJ, Whang KS, et al. Roseomonas aerophila sp. nov., isolated from air. Int J Syst Evol Microbiol 2013;63:2334-7.
3. Furuhata K, Ishizaki N, Edagawa A, Fukuyama M. Roseomonas tokyonensis sp. nov. isolated from a biofilm sample obtained from a cooling tower in Tokyo, Japan. Biocontrol Sci 2013;18:205-9.
4. Kim YK, Moon JS, Song KE, Lee WK. Two cases of bacteremia due to Roseomonas mucosa. Ann Lab Med 2016;36:367-70.
5. Dé I, Rolston KV, Han XY. Clinical significance of Roseomonas species isolated from catheter and blood samples: analysis of 36 cases in patients with cancer. Clin Infect Dis 2004;38:1579-84.
6. Kim KY, Hur J, Jo W, Hong J, Cho OH, Kang DH, et al. Infectious spondylitis with bacteremia caused by Roseomonas mucosa in an immunocompetent patient. Infect Chemother 2015;47:194-6.
7. Bibashi E, Sofianou D, Kontopoulou K, Mitsopoulos E, Kokolina E. Peritonitis due to Roseomonas fauriae in a patient undergoing continuous ambulatory peritoneal dialysis. J Clin Microbiol 2000;38:456-7.
8. Struthers M, Wong J, Janda JM. An initial appraisal of the clinical significance of Roseomonas species associated with human infections. Clin Infect Dis 1996;23:729-33.
9. Clinical and Laboratory Standards Institute (CLSI). Interpretive criteria for identification of bacteria and fungi by targeted DNA sequencing; approved guideline MM18-ED2. Wayne; PA:2018.
10. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; approved guideline M100-ED30. Wayne; PA:2019.