Departments of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
Corresponding to Mi-Na Kim, E-mail: mnkim@amc.seoul.kr
Ann Clin Microbiol 2022;25(3):103-107. https://doi.org/10.5145/ACM.2022.25.3.6
Received on 4 July 2022, Revised on 31 August 2022, Accepted on 31 August 2022, Published on 20 September 2022.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Dear Editor,
Since the first confirmed case of coronavirus disease 2019 (COVID-19) on January 20, 2020, a total of 719,269 COVID-19 patients have been confirmed in Korea. A total of 10,874 cases were currently treated under quarantine in January 21, 2022. As of August 7, 2022, the number of COVID-19 patients counted is 20,489,128 [1]. Until January 26, 2022, there were two kinds of the criteria for releasing the confirmed case from quarantine; first, two negative SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR) consecutively tested with interval of 24 hours and longer and symptomatic improvement. Second, at least 24 hours or longer fever-free period 10 days later after diagnosis [2]. Because Korea Disease Control and Prevention Agency (KDCA) adapted 3T policy of test-track-treat, all confirmed cases were considered infectious, they were always treated under quarantine, and trigger labor-intensive investigation to search contact persons [3]. However, as COVID-19 pandemic is prolonged more than 2 years, more patients newly diagnosed are in the convalescent period and non-infectious. Infection control to prevent spread in hospital spends huge resource of healthcare system. This study aimed to evaluate to estimate the prevalence of the patient at convalescent period among the newly diagnosed patients and suggest laboratory indicate to differentiate non-infectious patients.
SARS-CoV-2 RT-PCR test results were retrospectively reviewed in Asan Medical Center from February 2020 to September 2021. RT-PCR was performed using Allplex 2019-nCoV Assay (Seegene, Seoul, Korea) and STANDARD M n-CoV real-time Detection Kit (SD Biosensor, Suwon, Korea). For SARS-CoV-2 antibody test, STANDARD Q COVID-19 IgM/IgG Plus Test (SD Biosensor, Inc., Suwon, Korea) was used. If either IgM or IgG was positive, it was interpreted as antibody positive, that is, past infection status.
Of the total 678 patients, 233 (34.3%) had initial cycle threshold (Ct) values of 30 or higher. Among them, 164 (70.4%) cases were confirmed as COVID-19 patients by other institutions or the persons with past COVID-19 infections. A total of 69 (29.6%) cases were newly confirmed at our hospital, and two of them were already vaccinated and were excluded from the antibody test. A total of 32 patients underwent SARSCoV-2 antibody tests within 4 days, and 27 of them followed by PCR tests. A total of 31 patients, including four patients who did not receive antibody testing, were monitored for PCR testing after diagnosis. 23 (71.9%) patients were antibody-positive, and all but four patients who were not subjected to follow-up PCR testing repeatedly showed high Ct values or converted to negative within 2 days, indicating that they were in the convalescent period. All nine antibody-negative patients except for one without follow-up PCR test showed the follow-up Ct values of less than 20, thus they were in early infection period. Two out of four patients who were not tested for antibody but followed up by PCR testing showed the Ct value less than 20, thus suggested early infection period. The remaining two patients, consisting of one negative in PCR test and one having a repeated high Ct value for 14 days, were considered convalescent (Fig. 1). Among the 31 patients who followed the PCR test more than twice, the trend of Ct values in 11 (35.5%) patients was at 30 or higher at f irst and then the Ct values gradually decreased, which was estimated to be early infection period (Fig. 2A) Therefore, the Ct value of 30 or higher is more likely to be convalescent period rather than early infection period. Especially, this can be probed when antibody is positive or follow-up Ct is not getting lowered. However the certain patient with Ct of 30 or higher could be in early infection period. According to the COVID-19 severity scale in the clinical treatment guideline presented by KDCA, three patients were mild and seven were severe when applied to patients in the early infection category. In contrast, the convalescent patients consisted of 2 asymptomatic (9.5%), 14 mild (66.6%), 3 severe (14.2%), and 2 unevaluable (9.5%) patients (Fig. 2B). Severe cases were defined as those with radiographic findings of pneumonia and requiring medical treatment [4]. Therefore, convalescent patients mainly were mild or asymptomatic COVID-19 patients.
In conclusion, it is important to distinguish the convalescent and early infection period of COVID-19 in order to reduce unnecessary isolation. Therefore, if the initial Ct value in SARS-CoV-2 RT-PCR is 30 or higher, additional tests are needed to confirm the infectivity of the patient. Antibody positivity and repeatedly high Ct value 1 or 2 days later is useful laboratory findings to indicate convalescent period.
A limitation of this study is that the size of the subjects is small. At the time of this study, there were relatively few COVID-19 patients compared to the delta and omicron variant outbreak period in Korea. However it is now impossible to conduct more large-scale studies, because antibody positivity is no longer an indicator of the convalescent period after infection when the vaccination rate is high as it is now. Second, monitoring the PCR test until negative for release from isolation is no longer in use, so it is difficult to assess the trend of high Ct values. Instead, since anti-N antibodies are formed only by infection, they can be distinguished from immunity by vaccination, which can be useful when the Ct value is high at initial diagnosis.
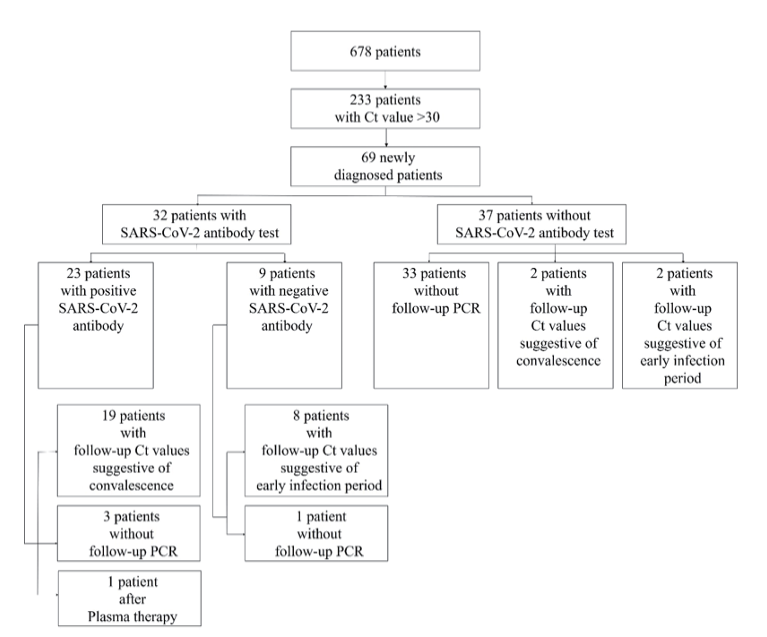
Fig. 1. Flowchart determining convalescence and early infection in newly diagnosed SARS-CoV-2 cases by SARS-CoV-2 PCR and antibody tests. Ct, cycle of threshold; PCR, polymerase chain reaction.
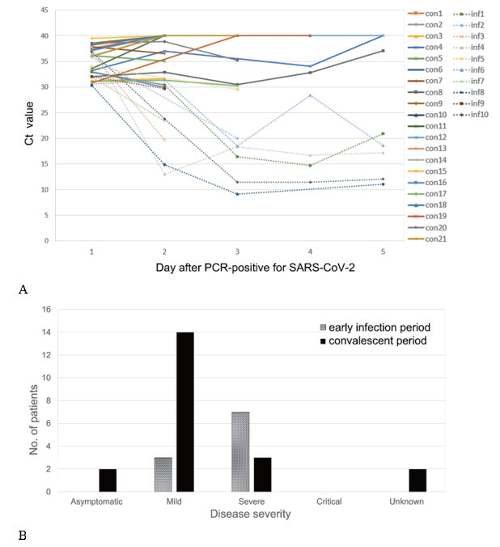
Fig. 2. Trends of Ct values of 31 patients with follow-up SARS-CoV2-PCR tests (A) and comparison of the severity of COVID-19 between patients in convalescent period and patients in early infection period (B). The trend lines of the recovery period were indicated by solid lines, and the trend lines of the early infection period were indicated by dotted lines. Ct, cycle of threshold; PCR, polymerase chain reaction.
This study was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea and was deemed to be exempt from informed consent (IRB No. 2022-1504-0001).
No potential conflicts of interest relevant to this article were reported.
None.
1. Korea Disease Control and Prevention Agency (KDCA). The updates on COVID-19 in Korea as of 21 January. http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_ id=&gubun [Online] (last visited on 8 August 2022).
2. Korea Disease Control and Prevention Agency (KDCA). Patient treatment and management. http://ncov.mohw.go.kr/baroView3.do?brdId=4&brdGubun=43 [Online] (last visited on 8 August 2022).
3. Centers for Disease Control and Prevention (CDC). COVID-19; Quarantine and isolation. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html [Online] (last visited on 8 August 2022).
4. Korea Disease Control and Prevention Agency (KDCA). Advice and notice. http://ncov.mohw. go.kr/upload/ncov/file/202003/1583112616462_20200302103016 [Online] (last visited on 1 September 2022).
1. Korea Disease Control and Prevention Agency (KDCA). The updates on COVID-19 in Korea as of 21 January. http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_ id=&gubun [Online] (last visited on 8 August 2022).
2. Korea Disease Control and Prevention Agency (KDCA). Patient treatment and management. http://ncov.mohw.go.kr/baroView3.do?brdId=4&brdGubun=43 [Online] (last visited on 8 August 2022).
3. Centers for Disease Control and Prevention (CDC). COVID-19; Quarantine and isolation. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html [Online] (last visited on 8 August 2022).
4. Korea Disease Control and Prevention Agency (KDCA). Advice and notice. http://ncov.mohw. go.kr/upload/ncov/file/202003/1583112616462_20200302103016 [Online] (last visited on 1 September 2022).