Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
Corresponding to Mi-Na Kim, E-mail: mnkim@amc.seoul.kr
Ann Clin Microbiol 2024;27(3):205-214. https://doi.org/10.5145/ACM.2024.27.3.4
Received on 13 August 2024, Revised on 30 August 2024, Accepted on 3 September 2024, Published on 20 September 2024.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Background: Unlike the Mycoplasma IST2 kit (bioMérieux), the Mycoplasma IST3 kit has been updated to comply with the standardized antimicrobial susceptibility test (AST) method for Ureaplasma spp. (Up) and Mycoplasma hominis (Mh). We aimed to verify the use of the Mycoplasma IST3 kit for genital mycoplasma cultures.
Methods: From September 2023 to January 2024, the R1 medium remaining after inoculation with IST2 was refrigerated until the next day. For IST2-positive samples, 300 μL of residual R1 medium was inoculated into the IST3. Species identification, enumeration, and AST results obtained using IST3 were compared with those obtained using IST2.
Results: A total of 48 IST2-positive samples were inoculated into IST3, including 35, 1, and 12 Up-only, Mh-only, and both Up- and Mh-positive samples, respectively. Among Up-only samples, 2.8%, 91.4%, and 100.0% were susceptible to ciprofloxacin, tetracycline, and erythromycin, respectively. With IST3, 45 (93.8%) samples grew genital mycoplasmas; 42 (89.4%) of the 47 Up-positive samples and 6 (46.2%) of the 13 Mh-positive samples showed growth of the same organisms. All seven samples that failed to grow Mh were from mixed cultures, of which four Mh concentrations of < 104/mL. Up was susceptible to levofloxacin, tetracycline, and erythromycin at the rates of 64.3 %, 88.1 %, and 95.2 %, respectively.
Conclusion: IST3 showed good performance in detecting genital Mycoplasma except for its tendency to not detect Mh of low concentrations in mixed cultures. IST3 is preferable to IST2 because it can accurately screen for erythromycin resistance in Up and reduce false-resistances for fluoroquinolone.
Antimicrobial, Culture, Genital, Mycoplasma, Ureaplasma
Mycoplasma and Ureaplasma (Up) species are the smallest bacteria with a diameter of 0.2–0.3 μm and are fastidious [1]. Mycoplasma hominis (Mh), Ureaplasma urealyticum, and Ureaplasma parvum are genital mycoplasmas that colonize up to 80% of sexually mature women and may cause genital infections from asymptomatic infections of the upper genital tract to endometritis, chorioamnionitis, and adverse pregnancy outcomes [1-4]. Therefore, it is important to differentiate true pathogens from colonizers when genital mycoplasmas are detected in nonsterile urogenital specimens [5,6]. Tetracyclines and fluoroquinolones are often used to treat patients. However, the resistance to common antimicrobial agents is increasingly reported [7,8]. The Mycoplasma IST assay (bioMérieux) is a culture-based diagnostic kit for species identification with enumeration and antimicrobial susceptibility tests (ASTs) to diagnose urogenital mycoplasma infections [9,10]. Since the introduction of the international standardization of antimicrobial breakpoints by the Clinical and Laboratory Standards Institute (CLSI) in 2011 [11], a novel version of IST, IST3, has been developed based on CLSI criteria for antimicrobial selection and concentration specific to Mh and Up. Unlike the previous version IST2 (bioMérieux), the IST3 assay is designed to independently test antimicrobial susceptibility for Mh and Up, even in mixed infections [10]. One publication on performance evaluation of the IST3 kit is available [12]; however, a parallel comparison study with IST2 is lacking.
The purpose of this study was to verify the performance of the Mycoplasma IST3 kit for cultures and AST of genital mycoplasmas compared to that of IST2.
It is a parallel study to verify the performance characteristics of the mycoplasma culture kit compared to the present version before introducing the new kit in the clinical laboratory. Therefore, diagnostic accuracy and precision were not evaluated.
Residual specimens from positive samples tested for cultures and AST of genital mycoplasmas at Asan Medical Center from September 2023 to January 2024, which were anonymized, were included in the study.
Residual R1 medium after inoculation with the IST2 kit was stored in a refrigerator until the following day, from September 2023 to January 2024. For the collection of culture-positive samples, in which LYO 2 broth became red after 24 h of incubation, 300 μL of residual R1 medium was inoculated into the IST3 kit at a 1:10 (R1:R2) dilution in R2 medium Both kits were read after 24 and 48 h of incubation, as recommended by the manufacturer [9,10]. IST2 contained one concentration well each of ≥ 104 color-changing units (CCU) for quantitation of Up and Mh. AST cupules contained doxycycline, josamycin, ofloxacin, erythromycin, tetracycline, ciprofloxacin, azithromycin, clarithromycin, and pristinamycin and did not distinguish between Mh and Up when mixed. IST3 contained three concentration wells of ≥ 103 CCU, ≥ 104 CCU, and ≥ 106 CCU for quantitation of Mh and two concentration wells of ≥ 104 CCU and ≥ 106 CCU for quantitation of Up. Susceptibilities to levofloxacin, moxifloxacin, tetracycline, erythromycin, and telithromycin were tested for Up and to levofloxacin, moxifloxacin, tetracycline, and clindamycin for Mh (Fig. 1). Because Mh possesses intrinsic macrolide resistance and was mostly mixed with Up, cumulative susceptibility was calculated by dividing the positive samples into two groups: all Mh-positive (Mh+) samples, including Mh-only positive samples, and Up-only positive (Up-only) samples. The AST results of IST2 were interpreted the manufacturer-recommended breakpoints except for the macrolide-intermediate, which was shifted to a susceptibility category, in line with current standard guidelines [11]: ofloxacin ≤ 1 μg/mL, ciprofloxacin ≤ 1 μg/mL, doxycycline ≤ 4 μg/mL, tetracycline ≤ 4 μg/mL, erythromycin ≤ 8 μg/mL, azithromycin ≤ 2 μg/ mL, clarithromycin ≤ 4 μg/mL and pristinamycin ≤ 2 μg/mL, and josamycin ≤ 2 μg/mL [9]. With the IST3 test, the cumulative susceptibilities of Mh- and Up-positive samples were calculated based on the CLSI breakpoints [11]: for Mh, levofloxacin ≤ 1 μg/mL, moxifloxacin ≤ 0.25 μg/mL, tetracycline ≤ 2 μg/mL, and clindamycin ≤ 0.25 μg/mL and for Up, levofloxacin ≤ 2 μg/mL, moxifloxacin ≤ 2 μg/mL, tetracycline ≤ 1 μg/mL, erythromycin ≤ 8 μg/mL, and telithromycin ≤ 4 μg/mL [10]. The AST results were not interpreted if all cupules changed to red.
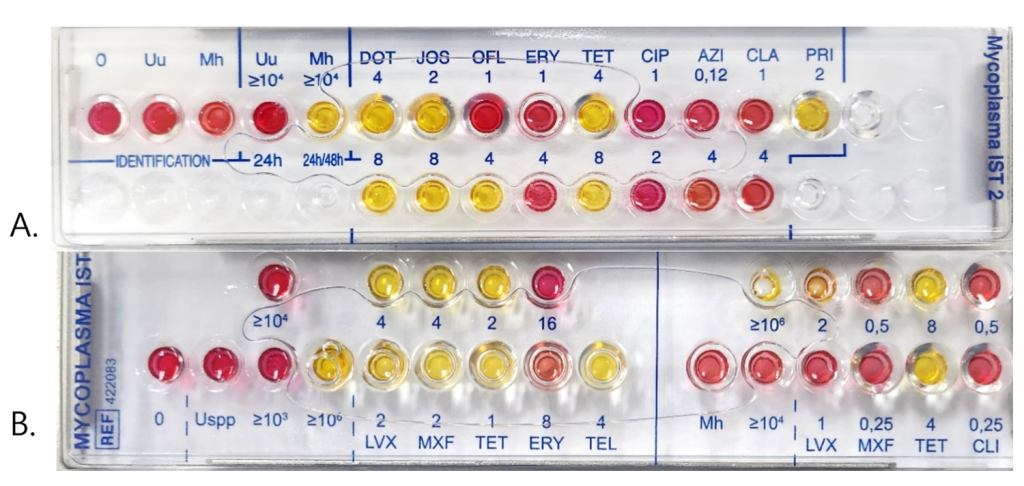
We compared the species, counts, and AST results of the two kits. Because the tested antimicrobials and their concentrations differed between the two kits, very major errors (VME), major errors (ME), and minor errors in IST3 were assessed by comparing the susceptibilities of Mh among Mh+ samples of IST2 and by comparing the susceptibilities of Up among Up-only samples of IST2 at the antimicrobial class level. Therefore, the error rates were calculated for fluoroquinolone classes, tetracycline, and macrolide classes for Up and fluoroquinolone classes and tetracycline for Mh.
A total of 48 IST2-positive samples were inoculated into IST3: 35 Up-only samples, 1 Mh-positive sample, and 12 samples positive for both Up and Mh.
Table 1. Evaluation of the performance of the Mycoplasma IST3 kit for analysis of speciation and enumeration of genital mycoplasmas compared to the Mycoplasma IST2 kit
| CCU by Mycoplasma IST2 | CCU by Mycoplasma IST3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Up ≥ 106 | Up ≥ 104 | Mh ≥ 104 & Up ≥104 | Mh < 104 & Up ≥104 | Up ≥ 103 | Up < 103 | Mh ≥ 106 | Mh ≥ 104 | No growth | Subtotal | |
| Up ≥ 104 | 5 | 23 | 1 | 3 | 2 | 34 | ||||
| Mh < 104 & Up ≥104 | 4 | 3a | 1 | 8 | ||||||
| Mh ≥ 104 & Up ≥104 | 2a | 1 | 1 | 4 | ||||||
| Up < 104 | 1 | 1 | ||||||||
| Mh < 104 | 1 | 1 | ||||||||
| Subtotal | 5 | 29 | 3 | 1 | 2 | 3 | 1 | 1 | 3 | 48 |
aFor both IST2 and IST3, one sample in each kit resulted in a color change to red in all cupules, and for these samples, the results for that kit were assigned as Mh ≥ 104 and Up ≥ 104.
Abbreviations: CCU, color-changing units; Up, Ureaplasma species; Mh, Mycoplasma hominis.
Table 2. Cumulative antimicrobial susceptibility of Mycoplasma hominis and Ureaplasma species tested using the Mycoplasma IST2 and Mycoplasma IST3 kits
| IST2 Antimicrobials | % Susceptibility | IST3 Antimicrobials | % Susceptibility | ||
|---|---|---|---|---|---|
| Mh+ (n=12) | Up (n=35) | Mh (n=5) | Up (n=42) | ||
| Ofloxacin | 8.3 | 17.1 | Levofloxacin | 60.0 | 64.3 |
| Ciprofloxacin | 8.3 | 2.8 | Moxifloxacin | 60.0 | 97.6 |
| Doxycycline | 91.6 | 91.4 | Clindamycin | 60.0 | – |
| Tetracycline | 91.6 | 91.4 | Tetracycline | 100.0 | 88.1 |
| Erythromycin | 0.0 | 100.0 | Erythromycin | 95.2 | – |
| Azithromycin | 0.0 | 100.0 | Telithromycin | – | 97.6 |
| Clarithromycin | 0.0 | 100.0 | – | – | – |
| Pristinamycin | 91.6 | 100.0 | – | – | – |
| Josamycin | 50.0 | 100.0 | – | – | – |
Abbreviations: Mh+; Mycoplasma hominis-positive samples including mixed cultures; Mh, Mycoplasma hominis; Up, Ureaplasma species.
Eight of the nine VME for fluoroquinolones in IST3 occurred in the Up-only group, with an ofloxacin MIC of 4 μg/mL (intermediate). One Up-only sample showed an ME for an ofloxacin MIC of 4 μg/mL and ciprofloxacin MIC of 2 μg/mL, tested by IST2, but for levofloxacin > 2 μg/mL and moxifloxacin ≤ 2 μg/mL when tested by IST3 (Table 3). Two out of five Mh samples were resistant to fluoroquinolones and clindamycin and all were susceptible to tetracycline. There was no mismatch in AST of Mh between IST2 and IST3.
Table 3. Summary of the mismatch results for fluoroquinolone susceptibility for 34 Ureaplasma species-positive samples using the Mycoplasma IST2 and IST3 kits
| Specimen No. | Fluoroquinolone MIC (μg/mL) | |||
|---|---|---|---|---|
| Ofloxacin | Ciprofloxacin | Levofloxacin | Moxifloxacin | |
| 8 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 10 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 17 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 19 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 22 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 29 | 4 | 2 | > 2 | ≤ 2 |
| 30 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 42 | 4 | > 2 | ≤ 2 | ≤ 2 |
| 45 | > 4 | > 2 | ≤ 2 | ≤ 2 |
| 47 | 4 | > 2 | ≤ 2 | ≤ 2 |
Ofloxacin and ciprofloxacin were tested using the IST2 kit, whereas levofloxacin and moxifloxacin were tested using the IST3 kit. Abbreviation: MIC, minimum inhibitory concentration.
The positive results for IST3 correlated well with those for IST2. The IST2-positive samples were also positive with IST3 in 93.8% of samples. The Up-positive samples (10.6%) in which Up failed to grow again, all contained initial concentrations of Up ≥ 104 CCU as enumerated by IST2. Possible causes for this failure in growth may be the loss of viability during storage or the one-tenth dilution of the specimens. Considering that the manufacturer recommends refrigerated storage of samples suspended in R1 media for up to 48 h for IST2 and up to 72 h for IST3 [9,10], the 24-h storage is unlikely to have resulted in deterioration in the viability of Up or Mh. Therefore, sample dilution is more likely to be the cause of the observed failure in growth. When tested with IST2, Mh tended to be identified as mixed with Up, and less than half of these samples were cultured successfully by IST3. A previous study evaluating IST3 showed high sensitivity and specificity for both Up and Mh [12]. In that study, the sensitivity and specificity for Mh was 95.7% and 100%, respectively, compared to that of the standard culture using A7 agar. The results of the present study raise the possibility of false positives for Mh in IST2, because it is difficult to explain the high rate of growth failure with IST3. After introducing IST3 into the laboratory, the overall culture positivity rate remained at 24%–25% over the previous 3 months without significant changes; however, mixed infections positive for both Up and Mh among all positive samples decreased from 14.4% to 1.9%, and the proportion of Mh-positive samples also decreased from 15.5% to 1.9% (unpublished data). Polymerase chain reaction (PCR)-based kits detect genital mycoplasmas more than twice as frequently as with IST [13,14]. Up accounted for 30%, whereas Mh and Mycoplasma genitalium accounted for 3.1% of all PCR-positive sexually transmitted pathogens in Korea [13]. Considering the ratio of Up and Mh by PCR, a significant proportion of mixed infections of Mh and Up may be false positives for Mh owing to the high load of Up, which may overcome the inhibitory effects in Mh-enumerating cupules in IST2.
The enumeration of Up with IST2 was reproducible with IST3 as 82.2% of Up samples with a load ≥ 104 CCU were also identified as having a load ≥ 104 CCU in IST3. This result was comparable to that of a previous study that compared IST3 to standard agar culture and reported an agreement of 83.7% for M. hominis and 86.3% for Up [12]. The change in bacterial load in three Up-only samples from ≥ 104 CCU to < 103 CCU and growth failure in four Up samples can be explained by the sample dilution effect. To date, no studies have compared IST2 and IST3 in parallel, as most specimens are swabs transported in R1 medium, leaving no equivalent specimens for repeat testing. In the present study, the R1 broth refrigerated after inoculation was successfully used for parallel testing to compare the performance of the old and new versions of the IST assay kit. It is necessary to perform parallel tests using patient samples when a new culture system is introduced to assess the performance specifications of the new system in a clinical laboratory. The residual R1 transport broth is a reasonable alternative to the swab itself.
When the performance of IST3 for ASTs in comparison to that of IST2 was evaluated for tetracycline and macrolide susceptibility of Up-only samples, two mismatches were observed: one ME and one VME in tetracycline. The occurrence of one VME was defined by the categorical interpretation results of each of the two systems; however, the MIC results were not discordant because the tetracycline concentrations tested were different. The result of one ME was from a sample that was diluted from ≥ 104 to ≥ 103 CCU, and the tetracycline MIC changed from > 8 μg/mL to 2 μg/mL. This ME was probably caused by a decrease in the bacterial load, which was inadequate to detect tetracycline resistance. Therefore, parallel testing using the residual R1 medium carries the risk of VME owing to sample dilution when the bacterial load in the original sample is close to 104 CCU. All five Mh samples tested were susceptible to tetracycline, which is consistent with the results of IST2. Tetracycline and doxycycline susceptibilities vary geographically [7,8], and in Korea, tetracycline susceptibility is reported to be 71.4%–100% and 81.0%–96.2% for Mh and Up, respectively [3,15-17]. The tetracycline susceptibilities in this study were consistent with those reported in previous studies. However, two erythromycin-resistant Up samples were found in two mixed infection samples with IST3. Macrolide resistance is often considered as a contributor to Mh in mixed cultures. Furthermore, one of the erythromycin-resistant Up samples was also resistant to telithromycin, which suggests acquired resistance to macrolide-lincosamide-streptogramin-ketolide class drugs, because telithromycin has not been used in Korea. Erythromycin is the primary drug for AST of Up, but macrolide resistance due to mutations in 23 rRNA and L22 ribosomal proteins is not rare in Korea [15]. The erythromycin AST is essential, especially in neonatal infections, as shown by a fatal case of erythromycin-resistant Ureaplasma meningitis in a premature infant treated with erythromycin in Korea [18]. The IST2 kit overestimates resistance in a mixed cultures containing Mh, which is inherently resistant to macrolides, and Up, which is inherently resistant to lincosamides [12]. Therefore, IST3 has a great advantage in that it can be used to independently test the AST of Mh and Up, even in mixed infections. IST2 also lacks the ability to determine whether the bacterial load, internationally set between 104 and 105 CCU, is compliant with the resistance threshold concentrations validated by the CLSI [11]. Therefore, nine VME and one ME of fluoroquinolones appeared to occur because IST2 used drugs that are less active than the standardized fluoroquinolones to test for levofloxacin and moxifloxacin. The susceptibility rates for ofloxacin and ciprofloxacin (17.1% and 2.8%, respectively) were similar to those of a previous study that collected the largest-scale data using IST2 over 3 years from 2016 to 2018 in Korea, showing susceptibilities of 18.7% and 6.0% for ofloxacin and ciprofloxacin, respectively [17]. Sequence analysis of gyrA, gyrB, parC, and parE detected amino acid mutations in only half of the ciprofloxacin-resistant Ureaplasma parvum isolates tested using IST2 [15]. Therefore, susceptibility of Up to fluoroquinolone seems to be biased toward greater resistance with IST2 [8,17]. Unlike conventional AST, which uses cultured bacteria, IST uses direct samples. Standardized AST for genital mycoplasmas is needed not only for patient care but also for monitoring the regional epidemiology of acquired antimicrobial resistance [17].
First, this study was designed as a parallel test using residual R1 broth instead of swab specimens. Furthermore, R1 broth was stored at 4℃ for 24 h and then used with a one tenth dilution. Therefore, when the culture was repeated with IST3 using residual R1 medium, the reason for missing four Up- and six Mh-positive samples was inferred to be the dilution effect or false-positive Mh testing by IST2. Viability loss of genital mycoplamas in R1 medium after sample inoculation is unlikely because it should be stable for 72 h at 4℃ [10]. Second, a relatively small number of specimens were included in this study, and the lack of resistant specimens can be a challenge for the validation of AST. However, the aim was to verify the culture performance of IST3, and not to verify or validate the performance of AST. In this study, decreased resistance to fluoroquinolone was anticipated with IST3. Third, this study did not include negative samples from IST2, and no standard agar cultures were performed; therefore, clinical sensitivity and specificity could not be determined. Given these limitations, this study is the minimum verification required to demonstrate non-inferior performance to IST2 for the introduction of an updated version of the IST. This study presents a reasonable design for verifying a new method for genital mycoplasma culture and AST in clinical laboratories, considering that it has already been thoroughly validated for approval as an in vitro diagnostics [12].
IST3 has good performance in detecting genital mycoplasmas but tends to detect the less Mh in mixed cultures. The IST3 kit is preferable to the IST2 kit because it can accurately screen for erythromycin resistance in Up and reduce false fluoroquinolone resistance.
Approval from the Institutional Review Board of Asan Medical Center was exempted because this study (2024-1037) was conducted to verify the in vitro diagnostic kit through parallel testing with the previous version.
Eun Jeong Won has been an associate editor and Heungsup Sung has been an editorial board member of the Annals of Clinical Microbiology since January 2024. However, they were not involved in the review process of this article. No other potential conflicts of interest relevant to this article were reported.
None.
The datasets generated during the current study are available for 1 year from the corresponding author.
1. Waites KB, Bébéar C. Mycoplasma and Ureaplasma. In: Caroll KC, et al. eds. Manual of Clinical Microbiology. 13th ed. Washington DC; American Society of Microbiology: 2023.
2. Chang YS, Kim SG, Kim BI, Park WS, Yoon BH, Kim EC, et al. Genital mycoplasma in the newborn infants: colonization, prevalence and clinical significance. J Korean Pediatr Soc 1996;30:1084-94.
3. Koh E, Kim S, Kim IS, Maeng KY, Lee SA. Antimicrobial susceptibilities of Ureaplasma urealyticum and Mycoplasma hominis in pregnant women. Korean J Clin Microbiol 2009;12:159-62.
4. Chung HK, Park SY, Park MH, Kim YJ, Chun SH, Cho SJ, et al. Association of genital mycoplasmas infection in women who had preterm delivery and outcomes in premature infants. Korean J Obstet Gynecol 2012;55:158-65.
5. D’Inzeo T, Angelis G, Fiori B, Menchinelli G, Liotti FM, Morandotti GM. Comparison of Mycoplasma IES, Mycofast Revolution and Mycoplasma IST2 to detect genital mycoplasmas in clinical samples. J Infect Dev Ctries 2017;11:98-101.

6. Park HR, Kim YH, Lee HJ, Oh JS, Kim HJ. Usefulness of the Mycofast test (MYCOFASTⓇ Evolution 2) for the diagnosis of nongonococcal genitourinary infections. Korean J Urol 2006;47:1117-23.
7. Wen X, Nobakht MS, Yang Y, Kouhsari E, Hajilari S, Shakourzadeh MZ, et al. Tetracyclines resistance in Mycoplasma and Ureaplasma urogenital isolates derived from human: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2023;22:83.


8. Song J, Wu X, Kong Y, Jin H, Yang T, Xie X, et al. Prevalence and antibiotics resistance of Ureaplasma species and Mycoplasma hominis in Hangzhou, China, from 2013 to 2019. Front Microbiol 2022;13:982429.


9. Mycoplasma IST2. Diagnosis of urogenital mycoplasma (culture, identification, enumeration, and susceptibility testing). Package insert. bioMérieux REF 42505.
10. Mycoplasma IST3. Diagnosis of urogenital mycoplasma (culture, identification, enumeration, and susceptibility testing). Package insert. bioMérieux REF 422083.
11. Clinical and Laboratory Standards Institute. Methods for antimicrobial susceptibility testing for human mycoplasmas-first edition: M43-A. Wayne; CLSI: 2011.
12. Boostrom I, Bala Y, Vasic JM, Gluvakov J, Chanard E, Barratt AH. Evaluation of the Mycoplasma IST3 urogenital mycoplasma assay in an international multicentre trial. J Antimicrob Chemother 2021;76:3175-82.


13. Choe HS, Lee DS, Lee SJ, Hong SH, Park DC, Lee MK, et al. Performance of Anyplex™ II multiplex real-time PCR for the diagnosis of seven sexually transmitted infections: comparison with currently available methods. Int J Infect Dis 2013;17:e1134-40.

14. Min SK, Kim SK, Kim YS, Cho IC, Lee GI. Evaluation of clinical sample for Accupower UU Real-Time PCR kit. Korean J Urogenit Tract Infect Inflamm 2014;9:99-103.
15. Chang J, Yu JK, Song C, Park IY, Park YJ. Prevalence and antimicrobial susceptibility of genital Mycoplasmataceae in Korean women: correlation between phenotypic test and resistance genes. Ann Clin Microbiol 2016;19:13-9.
16. Kweon OJ, Lim YK, Oh SM, Kim TH, Choe HS, Lee SJ, et al. Prevalence and antimicrobial susceptibility of Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum in individuals with or without symptoms of genitourinary infections. Lab Med Online 2016;6:79-87.
1. Waites KB, Bébéar C. Mycoplasma and Ureaplasma. In: Caroll KC, et al. eds. Manual of Clinical Microbiology. 13th ed. Washington DC; American Society of Microbiology: 2023.
2. Chang YS, Kim SG, Kim BI, Park WS, Yoon BH, Kim EC, et al. Genital mycoplasma in the newborn infants: colonization, prevalence and clinical significance. J Korean Pediatr Soc 1996;30:1084-94.
3. Koh E, Kim S, Kim IS, Maeng KY, Lee SA. Antimicrobial susceptibilities of Ureaplasma urealyticum and Mycoplasma hominis in pregnant women. Korean J Clin Microbiol 2009;12:159-62.
4. Chung HK, Park SY, Park MH, Kim YJ, Chun SH, Cho SJ, et al. Association of genital mycoplasmas infection in women who had preterm delivery and outcomes in premature infants. Korean J Obstet Gynecol 2012;55:158-65.
5. D’Inzeo T, Angelis G, Fiori B, Menchinelli G, Liotti FM, Morandotti GM. Comparison of Mycoplasma IES, Mycofast Revolution and Mycoplasma IST2 to detect genital mycoplasmas in clinical samples. J Infect Dev Ctries 2017;11:98-101.
6. Park HR, Kim YH, Lee HJ, Oh JS, Kim HJ. Usefulness of the Mycofast test (MYCOFASTⓇ Evolution 2) for the diagnosis of nongonococcal genitourinary infections. Korean J Urol 2006;47:1117-23.
7. Wen X, Nobakht MS, Yang Y, Kouhsari E, Hajilari S, Shakourzadeh MZ, et al. Tetracyclines resistance in Mycoplasma and Ureaplasma urogenital isolates derived from human: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2023;22:83.
8. Song J, Wu X, Kong Y, Jin H, Yang T, Xie X, et al. Prevalence and antibiotics resistance of Ureaplasma species and Mycoplasma hominis in Hangzhou, China, from 2013 to 2019. Front Microbiol 2022;13:982429.
9. Mycoplasma IST2. Diagnosis of urogenital mycoplasma (culture, identification, enumeration, and susceptibility testing). Package insert. bioMérieux REF 42505.
10. Mycoplasma IST3. Diagnosis of urogenital mycoplasma (culture, identification, enumeration, and susceptibility testing). Package insert. bioMérieux REF 422083.
11. Clinical and Laboratory Standards Institute. Methods for antimicrobial susceptibility testing for human mycoplasmas-first edition: M43-A. Wayne; CLSI: 2011.
12. Boostrom I, Bala Y, Vasic JM, Gluvakov J, Chanard E, Barratt AH. Evaluation of the Mycoplasma IST3 urogenital mycoplasma assay in an international multicentre trial. J Antimicrob Chemother 2021;76:3175-82.
13. Choe HS, Lee DS, Lee SJ, Hong SH, Park DC, Lee MK, et al. Performance of Anyplex™ II multiplex real-time PCR for the diagnosis of seven sexually transmitted infections: comparison with currently available methods. Int J Infect Dis 2013;17:e1134-40.
14. Min SK, Kim SK, Kim YS, Cho IC, Lee GI. Evaluation of clinical sample for Accupower UU Real-Time PCR kit. Korean J Urogenit Tract Infect Inflamm 2014;9:99-103.
15. Chang J, Yu JK, Song C, Park IY, Park YJ. Prevalence and antimicrobial susceptibility of genital Mycoplasmataceae in Korean women: correlation between phenotypic test and resistance genes. Ann Clin Microbiol 2016;19:13-9.
16. Kweon OJ, Lim YK, Oh SM, Kim TH, Choe HS, Lee SJ, et al. Prevalence and antimicrobial susceptibility of Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum in individuals with or without symptoms of genitourinary infections. Lab Med Online 2016;6:79-87.