Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea
Corresponding to Mi-Kyung Lee, E-mail: cpworld@cau.ac.kr
Ann Clin Microbiol 2024;27(3):185-196. https://doi.org/10.5145/ACM.2024.27.3.6
Received on 2 February 2024, Revised on 8 September 2024, Accepted on 9 September 2024, Published on 20 September 2024.
Copyright © Korean Society of Clinical Microbiology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND) (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Background: As most Candida species cause opportunistic infections, it is helpful for patient care to determine species name of Candida spp. and their distribution in both sterile and non-sterile specimens. We aimed to investigate trends in the distribution of Candida species isolated from a hospital in Korea, along with their antifungal susceptibilities and seasonal variations.
Methods: This study was conducted at the Chung-Ang University Hospital and included 8,760 different clinical specimens from March 2011 to December 2022. Identification of the fungal species and its antifungal susceptibility testing were performed using VITEK 2 ID-YST system for six drugs: amphotericin B, caspofungin, fluconazole, voriconazole, micafungin, and flucytosine.
Results: The most common fungal species was Candida albicans. The C. albicans positivity rate gradually increased from 2012 to 2022. Since 2020, however, this trend reversed and the non-albicans Candida (NAC) superseded the count of C. albicans. Among the NAC, C. glabrata showed significant increase. When a weekly analysis was performed, C. glabrata was evenly distributed without any noticeable peak; however, the positive rate decreased from late December to early January across all years.
Conclusion: Monitoring of future trends should necessarily be continued. Our findings revealed that the positive rate for Candida was the lowest in the months of December and January of the studied years, which can be attributed to environmental factors. However, further research needs to be conducted.
Antifungal agents, Candida albicans, Epidemiology, Opportunistic infections, Republic of Korea
Globally, antimicrobial resistance is the leading cause of death due to microbial infections [1]. In 2015, the World Health Organization launched the Global Antimicrobial Resistance Surveillance and Use System (GLASS), which was the first global collaboration to monitor antimicrobial resistance [2]. GLASS presents epidemiological data to monitor the impact and degree of antimicrobial resistance in populations worldwide [2]. In 2019, with an increasing number of fungal infections becoming resistant to antifungal drugs, a protocol was developed for collecting data on the early detection of Candida species [3]. However, compared to bacteria, accurate identification of Candida species and testing their antifungal resistance is a challenge because numerous microbiological laboratories worldwide lack the facility for these measurements [3]. In Korea, the Korea Centers for Disease Control and Prevention launched a system (Kor-GLASS) in 2017 to monitor antimicrobial resistance [4]. Candida is one of the most common causes of hospital-acquired infections [5]. According to the 2021 National Antimicrobial Resistant Microbial Survey annual report published by Kor-GLASS, surveillance of Candida began in the year 2020; among the Candida isolates from blood collected in 2021 from centers in nine regions, 360 were hospital-acquired infections, and 50 cases were community-acquired infections [5].
Regarding domestic research in Korea, several reports have been published focusing on surveillance of the antifungal susceptibility of Candida identified in sterile specimens, especially blood [6–8]. However, reports monitoring the distribution of Candida species and their antifungal drug susceptibility in various clinical samples— such as oral, skin, and urine as well as rare aseptically collected samples— exist in Korea [9–11]. As most Candida species cause opportunistic infections, it is helpful to analyze the distribution of Candida species detected in sterile specimens like blood, and in nonsterile specimens like the human mucous membranes, to identify the infectious species and conduct epidemiological investigations [11]. The distribution of Candida species in the studied specimens varied depending on the region, hospital, and the type of specimen [12].
Several studies have reported seasonal changes in the incidence of various infectious diseases [13]. Multiple factors, including temperature, humidity, school term, eating habits, and the environment preferred by microorganisms, are responsible for seasonal changes in infectious diseases. Therefore, studies conducted across different seasons may reveal various seasonal patterns in the growth of different types of microorganism, and may exhibit multiple growth peaks during the year [13]. However, few studies have investigated the seasonality of Candida epidemiology, and even these were limited to either one species of Candida or to a fixed age group of the study participants [14,15]. Moreover, no reports of Candida seasonality have been published in Asian countries.
This study, conducted at the Chung-Ang University Hospital, investigated the distribution of fungal species and antifungal drug susceptibility in various clinical and sterile specimens to confirm the latest epidemiology of Candida and to obtain information for future Candida treatment in South Korea. We also analyzed seasonal variations in Candida by evaluating long-term data.
It is a retrospective surveillance study based on the laboratory records. It was described according to the Microbiology Investigation Criteria for Reporting Objectively: a framework for the reporting and interpretation of clinical microbiology data available at: https://bmcmedicine.biomedcentral.com/ articles/10.1186/s12916-019-1301-1.
This study was conducted at the Chung-Ang University Hospital and targeted 8,760 different clinical specimens, including both sterile and non-sterile specimens, from patients who visited the hospital over a period of 12 years from March 2011 to December 2022. Duplicate samples were excluded from the analyses. Sterile specimens included blood, ascites, pleural fluid, and amniotic fluid, while non-sterile specimens included respiratory specimens, urine, feces, vaginal discharge, abscess, pus, and catheter tips.
Species were identified using the VITEK 2 ID-YST system (bioMérieux, Inc.) according to the manufacturer’s instructions. Antifungal susceptibility testing was conducted using the VITEK ASTYS07 (bioMérieux, Inc.) card of the VITEK 2 ID-YST system for the following six drugs: amphotericin B, caspofungin, fluconazole, voriconazole, micafungin, and flucytosine until November, 2019. Antifungal susceptibility testing was conducted using a VITEK AST-YS08 card (bioMérieux, Inc.) of the VITEK 2 ID-YST system. The minimum inhibitory concentration (MIC) was determined according to the standards defined by the Clinical and Laboratory Standards Institute (CLSI), which are periodically updated.
The Department of Laboratory Medicine at Chung-Ang University College of Medicine has been participated in the External Quality Assurance/Proficiency Testing Program of the Korean Association of External Quality Assessment Service and has been accredited by the Outstanding Laboratory Accreditation Program of the Laboratory Medicine Foundation/Korean Society for Laboratory Medicine.
Duplicate and sequential isolates from the same patient were not consistently done.
Statistical analysis was performed using the chi-square test with MedCalc software v. 19.5.1 (MedCalc Software), and p < 0.05 was considered statistically significant. Weekly trend analysis was performed for 52 weeks over 11 years to determine Candida seasonality. The number of Candida species over the period of 52 weeks, calculated weekly, and the proportion of Candida species compared to all specimens requested for microbial identification at the Chung-Ang University Hospital were graphed for visual comparison.
The antifungal drug susceptibility test results for the 8,760 Candida strains are summarized in Table 1. The most common Candida species was C. albicans (4,226 strains, 48.2%), followed by C. tropicalis (2,083 strains, 23.8%), C. glabrata (1,445 strains, 16.5%), and C. parapsilosis (554 strains, 6.3%). The MICs of amphotericin B, caspofungin, flucytosine, fluconazole, micafungin, and voriconazole for the 8,760 strains of Candida were distributed in the range of 0.25–16 μg/mL, 0.125–8 μg/mL, 1–64 μg/mL, 0.25–64 μg/ mL, 0.06–8 μg/mL, and 0.125–8 μg/mL, respectively, with differences depending on the fungal species. C. tropicalis showed 100% sensitivity to amphotericin B; however, seven strains of C. albicans showed resistance, and one strain of C. glabrata, and four strains of C. parapsilosis showed intermediate resistance. C. albicans, C. tropicalis, and C. parapsilosis were 98.6%, 98.8%, and 97.8% sensitive to fluconazole, respectively. However, only 228 (15.8%) C. glabrata strains were sensitive to fluconazole, 779 (83.7%) strains showed intermediate resistance, and 8 strains (0.6%) showed resistance.
Table 1. Antifungal susceptibilities of 8,760 Candida isolates from clinical specimens determined using the VITEK AST-YS07,08 card
| Species | No. of isolates | Antifungal agent | MIC (µg/mL) Range | % of MICs by category | ||
|---|---|---|---|---|---|---|
| S | I/SDD | R | ||||
| C. albicans | 4226 | Amphotericin B | ≤0.25-8 | 99.8 | 0.0 | 0.2 |
| Caspofungin | ≤0.125-0.5 | 100.0 | 0.0 | 0.0 | ||
| Flucytosine | ≤1-≥64 | 94.7 | 0.1 | 5.2 | ||
| Fluconazole | ≤0.25-≥64 | 98.6 | 1.4 | 0.2 | ||
| Micafungin | ≤0.06-0.25 | 100.0 | 0.0 | 0.0 | ||
| Voriconazole | ≤0.125-≥8 | 99.1 | 0.1 | 0.8 | ||
| C. tropicalis | 2083 | Amphotericin B | ≤0.25-1 | 100.0 | 0.0 | 0.0 |
| Caspofungin | ≤0.125-≥8 | 99.6 | 0.0 | 0.4 | ||
| Flucytosine | ≤1-≥64 | 99.9 | 0.0 | 0.1 | ||
| Fluconazole | ≤0.5-≥64 | 98.8 | 0.5 | 0.8 | ||
| Micafungin | ≤0.06-2 | 99.6 | 0.1 | 0.4 | ||
| Voriconazole | ≤0.125-4 | 99.3 | 0.0 | 0.6 | ||
| C. glabrata | 1445 | Amphotericin B | ≤0.25-2 | 99.9 | 0.1 | 0.0 |
| Caspofungin | ≤0.125-4 | 42.3 | 43.2 | 14.6 | ||
| Flucytosine | ≤1-≥64 | 99.6 | 0.0 | 0.4 | ||
| Fluconazole | ≤0.5-≥64 | 15.8 | 83.7 | 0.6 | ||
| Micafungin | ≤0.06-0.5 | 99.8 | 0.0 | 0.2 | ||
| Voriconazole | ≤0.125-≥8 | – | – | – | ||
| C. parapsilosis | 554 | Amphotericin B | ≤0.25-2 | 99.3 | 0.7 | 0.0 |
| Caspofungin | 0.125-2 | 100.0 | 0.0 | 0.0 | ||
| Flucytosine | ≤1 | 100.0 | 0.0 | 0.0 | ||
| Fluconazole | ≤0.5-≥64 | 97.8 | 1.1 | 1.3 | ||
| Micafungin | ≤0.06-2 | 100.0 | 0.0 | 0.0 | ||
| Voriconazole | ≤0.125-≥8 | 99.1 | 0.4 | 0.5 | ||
| C. utilis | 92 | Amphotericin B | ≤0.25-≥16 | 98.9 | 0.0 | 1.1 |
| Caspofungin | ≤0.125-0.25 | 100.0 | 0.0 | 0.0 | ||
| Flucytosine | ≤1 | 100.0 | 0.0 | 0.0 | ||
| Fluconazole | ≤0.5-32 | 98.9 | 1.1 | 0.0 | ||
| Micafungin | ≤0.06-0.125 | 100.0 | 0.0 | 0.0 | ||
| Voriconazole | ≤0.125-0.5 | 100.0 | 0.0 | 0.0 | ||
| C. famata | 73 | Amphotericin B | ≤0.25-1 | 100.0 | 0.0 | 0.0 |
| Caspofungin | ≤0.25-1 | 100.0 | 0.0 | 0.0 | ||
| Flucytosine | ≤1 | 100.0 | 0.0 | 0.0 | ||
| Fluconazole | ≤1-32 | 98.6 | 1.4 | 0.0 | ||
| Micafungin | ≤0.06-0.125 | 100.0 | 0.0 | 0.0 | ||
| Voriconazole | ≤0.125-0.25 | 100.0 | 0.0 | 0.0 | ||
| C. kruseia | 73 | Amphotericin B | ≤0.25-2 | 90.8 | 9.2 | 0.0 |
| Caspofungin | ≤0.125-0.5 | 84.7 | 15.3 | 0.0 | ||
| Flucytosine | 2-16 | 4.1 | 4.1 | 91.8 | ||
| Fluconazole | 2-≥64 | 0.0 | 0.0 | 100.0 | ||
| Micafungin | ≤0.06-0.25 | 100.0 | 0.0 | 0.0 | ||
| Voriconazole | ≤0.125-0.25 | 100.0 | 0.0 | 0.0 | ||
| C. lusitaniae | 64 | Amphotericin B | ≤0.25-1 | 100.0 | 0.0 | 0.0 |
| Caspofungin | ≤0.25-0.5 | 100.0 | 0.0 | 0.0 | ||
| Flucytosine | ≤1 | 100.0 | 0.0 | 0.0 | ||
| Fluconazole | ≤0.5-8 | 100.0 | 0.0 | 0.0 | ||
| Micafungin | ≤0.06-0.5 | 100.0 | 0.0 | 0.0 | ||
| Voriconazole | ≤0.125 | 100.0 | 0.0 | 0.0 | ||
| Othersb | 150 | Amphotericin B | ≤0.25-≥16 | 91.6 | 1.4 | 7.0 |
| Caspofungin | ≤0.125-≥8 | 91.0 | 5.4 | 3.6 | ||
| Flucytosine | ≤1-≥64 | 96.2 | 3.0 | 0.8 | ||
| Fluconazole | ≤0.5-≥64 | 74.6 | 6.6 | 19.4 | ||
| Micafungin | ≤0.06-≥8 | 97.9 | 1.0 | 1.0 | ||
| Voriconazole | ≤0.125-4 | 91.8 | 7.4 | 0.8 | ||
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; SDD, susceptible dose-dependent; I, intermediate; R, resistant.
aC. krusei was resistant to fluconazole, irrespective of its MIC.
bOthers include C. haemulonii, C. orthopsiolosis, C. guilliermondii, C. kefyr, C. auris, C. ciferrii, C. norvegensis, C. lipolytica, and C. inconspicua.
Table 2 summarizes and compares the species distribution of Candida isolates in non-sterile and sterile specimens. Very rare samples and cases, where the sample type was not recorded, were excluded. A total of 1,351 Candida strains were isolated from sterile specimens, including blood, ascites, pleural fluid, and cerebrospinal fluid. The most commonly isolated fungal species from the total sterile body fluid specimens were C. albicans (673 strains, 49.8%), and C. glabrata (240 strains, 17.8%), followed by C. tropicalis (230 strains, 17.0%), C. parapsilosis (131 strains, 9.7%), and C. krusei (22 strains, 1.6%).
A total of 7,324 Candida strains were isolated from non-sterile specimens, including respiratory samples, urine, stool, and vaginal discharge; most of the commonly isolated strains were from urine samples (6,320 strains, 86.3%), followed by respiratory samples (338 strains, 4.6%). C. albicans was the most common species isolated from non-sterile specimens (3,483 strains, 47.6%), followed by C. tropicalis (1,823 strains, 24.9%), C. glabrata (1,187 strains, 16.2%), and C. parapsilosis (419 strains, 5.7%).
Table 2. Species distribution of Candida isolates from sterile and nonsterile clinical specimens
| Species | Respiratory | Pus/Wound | Urine | Stool | Tissue | Vaginal discharge | Cath tip | Non-sterile Total | Blood | Ascitic fluid | Pleural fluid | Other sterile fluidb | Sterile Total | Sterile vs. Non-sterile p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ear discharge | Eye discharge | Other pus/wound | ||||||||||||||
| C. albicans | 194 | 10 | 3 | 87 | 2,967 | 1 | 165 | 56 | 3,483 | 461 | 19 | 15 | 178 | 673 | 0.2725 | |
| C. tropicalis | 117 | 4 | 36 | 1,632 | 9 | 25 | 1,823 | 154 | 8 | 12 | 56 | 230 | < 0.0001a | |||
| C. glabrata | 4 | 1 | 13 | 1,131 | 2 | 23 | 13 | 1,187 | 206 | 12 | 6 | 16 | 240 | 0.2272 | ||
| C. parapsilosis | 2 | 108 | 4 | 19 | 271 | 1 | 14 | 419 | 123 | 1 | 7 | 131 | < 0.0001a | |||
| C. utilis | 1 | 1 | 85 | 1 | 88 | 4 | 4 | 0.0026a | ||||||||
| C. famata | 3 | 7 | 135 | 2 | 147 | 9 | 1 | 1 | 11 | 0.0021a | ||||||
| C. krusei | 11 | 2 | 31 | 3 | 47 | 4 | 1 | 12 | 5 | 22 | 0.0001a | |||||
| C. lusitaniae | 6 | 44 | 1 | 51 | 8 | 4 | 12 | 0.4218 | ||||||||
| C. haemulonii | 31 | 1 | 3 | 35 | 0 | 0 | – | |||||||||
| C. orthopsilosis | 3 | 9 | 1 | 13 | 11 | 11 | 0.0002a | |||||||||
| C. guilliermondii | 2 | 11 | 1 | 14 | 15 | 1 | 16 | < 0.0001a | ||||||||
| C. kefyr | 0 | 1 | 1 | – | ||||||||||||
| C. auris | 8 | 1 | 9 | 0 | 0 | – | ||||||||||
| C. ciferrii | 3 | 1 | 4 | 0 | 0 | – | ||||||||||
| C. norvegensis | 2 | 2 | 0 | 0 | – | |||||||||||
| C. lipolytica | 1 | 1 | 0 | 0 | – | |||||||||||
| C. inconspicua | 1 | 1 | 0 | 0 | – | |||||||||||
| Total | 338 | 178 | 8 | 158 | 6,320 | 1 | 3 | 203 | 115 | 7,324 | 996 | 42 | 47 | 266 | 1,351 | |
ap < 0.05.
bOther sterile fluids included bile, amniotic fluid, intraocular (vitreous and aqueous) fluid, homovac drainage, and percutaneous transhepatic biliary drainage.
Overall, C. albicans was the most commonly identified species in almost all samples, except pus. The most common non-albicans Candida species isolated from respiratory specimens was C. tropicalis whereas those isolated from urine and pus specimens were C. tropicalis and C. parapsilosis, respectively. In vaginal discharge, C. albicans was the most common species identified, followed by C. glabrata. Candida was the most common species with 996 strains isolated from blood, (73.7%), pleural fluid (3.5%), and ascitic fluid (3.1%).
To conduct the annual analysis, we used data from January 2012 to December 2022. During this period, in total, 697,410 samples were requested for microbial culture tests at the Department of Laboratory Medicine, Chung-Ang University Hospital, of which 8,533 (1.2%) tested positive for Candida. The Candida positivity rate gradually increased from 2012 (465 of 55,448 positive cases, 0.84 %) to 2022 (1,164 of 65,685 positive cases, 1.77%). In 2020, 1,282 positive cases out of 59,669 culture tests requested (2.15%) showed the highest positivity rate (Table 3).
Approximately 91% of the non-albicans Candida (NAC) comprised three species: C. tropicalis, C. glabrata, and C. parapsilosis. Although no change was observed in the occurrence of C. tropicalis, the most dominant fungus, C. glabrata demonstrated a significant increase in abundance from 2020 (Fig. 1). From 2012 to 2019, C. glabrata accounted for 13.9% of Candida cases (697 of 2,995 positive cases); however, the proportion increased from 2020 to 2022, accounting for 20% of Candida cases (708 of 3,538 positive cases).
Table 3. Yearly comparison analysis showing the epidemiological trend of Candida infection between 2012 and 2022
| Candida infection | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2012-2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida spp. | 465 | 401 | 414 | 615 | 794 | 550 | 802 | 954 | 1,282 | 1,092 | 1,164 | 8,533 |
| (0.84) | (0.66) | (0.67) | (0.95) | (1.20) | (0.94) | (1.13) | (1.36) | (2.15) | (1.71) | (1.77) | (1.22) | |
| Candida albicans | 216 | 216 | 207 | 267 | 403 | 243 | 387 | 519 | 577 | 503 | 540 | 4,078 |
| (46.45) | (53.87) | (50.00) | (43.41) | (50.76) | (44.18) | (48.25) | (54.40) | (45.01) | (46.06) | (46.39) | (47.79) | |
| NAC | 249 | 185 | 207 | 348 | 391 | 307 | 415 | 435 | 705 | 589 | 624 | 4,455 |
| (53.55) | (46.13) | (50.00) | (56.59) | (49.24) | (55.82) | (51.75) | (45.60) | (54.99) | (53.94) | (53.61) | (52.21) | |
| C. tropicalis | 41 | 61 | 94 | 139 | 198 | 191 | 244 | 207 | 294 | 251 | 248 | 1,968 |
| (8.82) | (15.21) | (22.71) | (22.60) | (24.94) | (34.73) | (30.42) | (21.70) | (22.93) | (22.99) | (21.31) | (23.06) | |
| C. glabrata | 94 | 34 | 34 | 135 | 118 | 66 | 73 | 143 | 272 | 210 | 226 | 1,405 |
| (20.22) | (8.48) | (8.21) | (21.95) | (14.86) | (12.00) | (9.10) | (14.99) | (21.22) | (19.23) | (19.42) | (16.47) | |
| C. parapsilosis | 23 | 23 | 21 | 44 | 38 | 17 | 69 | 45 | 88 | 74 | 99 | 541 |
| (4.95) | (5.74) | (5.07) | (7.15) | (4.79) | (3.09) | (8.60) | (4.72) | (6.86) | (6.78) | (8.51) | (6.34) | |
| Total | 55,448 | 60,344 | 61,735 | 64,943 | 66,428 | 58,371 | 71,027 | 69,998 | 59,669 | 63,762 | 65,685 | 697,410 |
Values are presented as n (%) or n.
In 2019, of the 954 positive cases for Candida, C. albicans and NAC accounted for 54.4 % (519 cases) and 45.6 % (435 cases) , respectively. C. albicans was identified significantly more frequently (p < 0.05). In 2020, this trend was reversed and significantly more NACs were identified. This trend is expected to continue after the year 2020.
Abbreviation: NAC, non-albicans Candida.
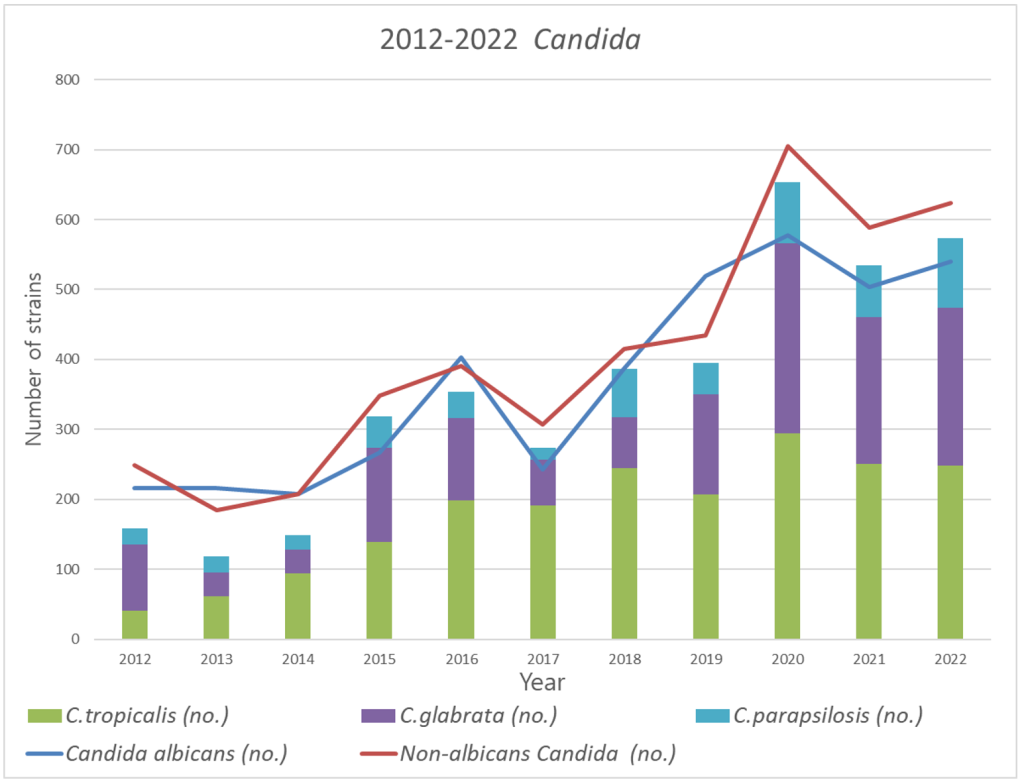
Fig. 1. Yearly analysis showing the number of Candida, including C. albicans, NAC, particularly C. tropicalis, C. glabrata, and C. parapsilosis, the three most predominant species of NAC. The Candida positivity rate gradually increased from 2012 (465 positive cases out of 55,448, 0.84%) to 2022 (1,164 positive cases out of 65,685 cases, 1.77%). C. albicans and NAC showed the same pattern. NAC, non-albicans Candida.
A weekly analysis performed for 52 weeks over 11 years revealed that the Candida positivity rate was the highest at week 44 (October 29 to November 4), followed by week 34 (August 20 to August 26) at 1.52%. It was lowest in week 1 at 0.91% (January 1 to January 7), followed by week 2 at 0.98 % (January 8 to January 14), week 22 at 0.98%, and week 52 at 0.99%. Fig. 2 shows the number of Candida positive cases per week and with respect to the ratio of the total number of samples received. Overall, it was evenly distributed without a noticeable peak; however, the positivity rate was generally low across all years from late December to early January.
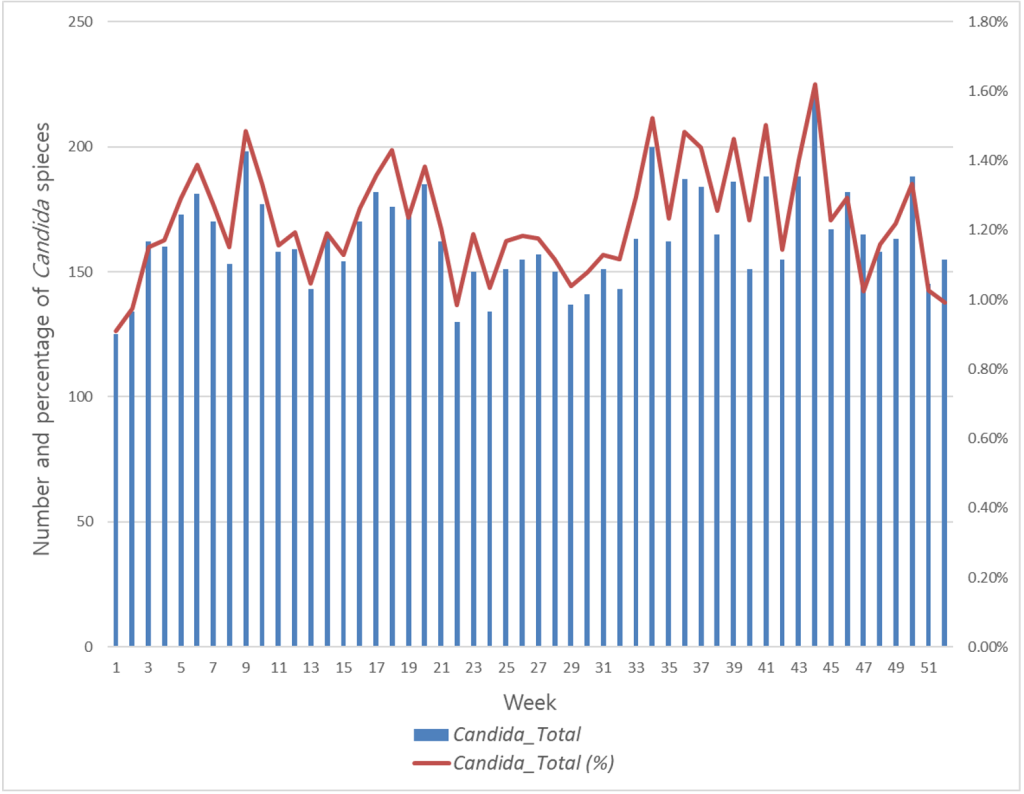
Fig. 2. Weekly comparison analysis showing the epidemiological trend of Candida infection between 2012 and 2022.
Fungal species with intrinsic resistance to antifungal drugs are well known; thus, major antifungal drug resistance information can be obtained by determining the distribution of fungal species [8]. Therefore, to appropriately treat fungal infections, periodic investigations of the distribution of the fungal species, isolated from clinical specimens in hospitals of relevant regions, and their antifungal drug resistance is necessary [8]. Among fungi, yeast is frequently isolated from clinical specimens that rapidly proliferate and form colonies similar to bacteria; thus, a standardized test for antifungal drug susceptibility has been developed and implemented in several laboratories [16]. In this study, the VITEK AST-YS07 and YS08 (bioMérieux, Inc.) of the VITEK 2 ID-YST system, an automated equipment that performs antifungal drug susceptibility testing, were used. Antifungal susceptibility testing using the VITEK 2 ID-YST system has been reported to possess a remarkably high agreement rate with the CLSI M27 method, a standardized test with good reproducibility [17,18]. The broth microdilution method, which is the reference method for the antifungal susceptibility testing, relies on visual MIC determination. The VITEK 2 ID-YST system uses spectrophotometry to determine the MIC endpoint, which eliminates subjectivity [17]. However, the VITEK 2 ID-YST system also minimizes the effects of trailing growth, compromising the performance of systems that rely on visual MIC determination [18].
Although antifungal susceptibility testing for yeast is not yet recommended as a routine test, the gradual increase in the incidence of severe infections caused by yeast, especially Candida spp., necessitates an increase for in vitro antifungal drug susceptibility testing [9]. In addition, in the United States, candidemia has gradually increased over the past 20 years, accounting for approximately 9% of nosocomial bloodstream infections and is the fourth most commonly isolated causative agent in the blood [19]. Candidemia or invasive Candida bloodstream infections are major causes of increased morbidity and mortality in patients [20]. Among fungi, > 90% of bloodstream infections are caused by Candida [21]. In Korea, C. albicans is the most common and clinically important cause of bloodstream infections, but NAC, including C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei, are also commonly identified in the order listed, and together they comprise the five most causative fungi of candidemia [22].
Although differences in the proportion of each Candida species exist among countries, similar trends in the increased use of antifungal drugs have been observed worldwide [23]. Comparing the epidemiology of Candida in Korea between 2001 and 2007 with reference to previously published reports [12], C. albicans accounted for 70%–93% of the total Candida spp. in the early 2000s, which significantly decreased to 44%54%, according to our data from 2012 to 2022.
After 2020, the NAC increased significantly, reversing the trend of C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis are the three representative species of the NAC. C. glabrata exhibited the most significant increase. More than 97% of strains of C. albicans, C. tropicalis, and C. parapsilosis were susceptible to fluconazole, whereas 84% of strains of C. glabrata were non-susceptible. The antifungal susceptibility of Candida isolates showed similar patterns between the early 2000s and the period of this study (2011–2022) [12]. However, the proportion of fluconazole-non-susceptible strains among C. glabrata significantly increased (5%–84%).
Thus, this finding is important for healthcare-related infections because C. glabrata is closely associated with antifungal drug resistance. Globally and in Korea, an increasing trend of C. glabrata has been observed [24], and a study showing similar results was published, although the timing of the study was different and only blood samples were used [25]. Therefore, continuous observation of future trends and antifungal drug resistance in Candida, especially C. glabrata is required.
There have been few reports on the seasonality of Candida. A report on the seasonality of invasive Candida infection in preterm low-birth-weight newborns revealed that infants were significantly more likely to be diagnosed with Candida infection between September and February [14]. The report attributed this observation to environmental factors, such as overcrowding. Another report investigating seasonal variation in vaginal Candida infection rates in Belgium [15] revealed that during summer, there was a 11% increase in the rate of vaginal presence of Candida. They observed a relationship between the frequency of C. albicans vaginitis and mean monthly temperature in the country, although this trend was not significant. Our results showed that the positivity rate of Candida was lowest in December and January, presumably influenced by environmental factors (e.g., temperature and humidity); however, this requires further research.
Some studies have shown that commercial microbial identification systems are unable to accurately identify C. auris [26]. In this study, two C. auris isolates were misidentified as C. albicans (confidence value 86.0) and C. haemulonii (confidence value 99.9) using VITEK 2 ID-YST system [26]. Therefore, it is necessary to confirm the Candida species identified as C. auris using additional tests. However, in this study, the statistical results were reported based on the results obtained using the VITEK 2 ID-YST system and additional tests were not performed. In addition, the clinical breakpoints for the antifungal drug susceptibility testing were revised during the course of this study; however, we did not provide a description or interpretation of these revisions. Data analysis was conducted based on the antifungal susceptibility results at the time the specimen was tested.
This study is significant because it is based on long-term data and includes all clinical samples. Based on the results obtained, if we can prospectively predict the prevalence of Candida, the use of prophylactic antifungal drugs in high-risk patients can reduce disease morbidity and severity. Although there are a few reports on the seasonality of Candida, there is no consistent conclusion, and as there has been no research in Asian countries, the observation of the seasonality of Candida in this study — low positive rate in December and January — is significant.
It is a retrospective surveillance study that includes only laboratory data with no clinical information; therefore, the obtainment of informed consent is not required.
Mi-Kyung Lee has been an ethics editor of the Annals of Clinical Microbiology since January 2024. However, she was not involved in the review process of this article. No other potential conflict of interest relevant to this article were reported.
We thank Beom Su Gu and the Microbiology Department of the Chung-Ang University Hospital for their contributions to this study.
None.
The datasets generated during the current study are available for 1 year from the corresponding author.
1. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399:629-55.
2. World Health Organization. Global antimicrobial resistance surveillance system (GLASS): technical meeting on the early implementation phase. 22-23 October 2015 WHO Regional Office for Europe Copenhagen, Denmark. Meeting Report. Geneva: WHO Press; 2016 Oct. Report No.: WHO/OHE/PED/AMR/2016.1.
3. World Health Organization. GLASS early implementation protocol for inclusion of Candida spp. https://www.who.int/publications/i/item/WHO-WSI-AMR-2019.4 [Online] (last visited on 11 January 2024).
4. Lee H, Yoon EJ, Kim D, Jeong SH, Shin JH, Shin JH, et al. Establishment of the South Korean national antimicrobial resistance surveillance system, Kor-GLASS, in 2016. Euro Surveill 2018;23:1700734.


5. Korea Disease Control and Prevention Agency. National Antimicrobial Resistance Surveillance in Korea, 2021. https://www.korea.kr/docViewer/skin/doc.html?fn=197089655&rs=/docViewer/result/2022.11/17/197089655 [Online] (last visited on 11 January 2024).
6. Won EJ, Choi MJ, Jeong SH, Kim D, Shin KS, Shin JH, et al. Nationwide surveillance of antifungal resistance of Candida bloodstream isolates in South Korean hospitals: two year report from Kor-GLASS. J Fungi 2022;8:996.


7. Won EJ, Shin JH, Choi MJ, Lee WG, Park YJ, Uh Y, et al. Antifungal susceptibilities of bloodstream isolates of Candida species from nine hospitals in Korea: application of new antifungal breakpoints and relationship to antifungal usage. PLoS One 2015;10:e0118770.


8. Won EJ, Shin JH, Lee WK, Koo SH, Kim SY, Park YJ, et al. Distribution of yeast and mold species isolated from clinical specimens at 12 hospitals in Korea during 2011. Ann Clin Microbiol 2013;16:92-100.
9. Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, et al. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev 2001;14:643-58.


10. Chae MJ, Shin JH, Cho D, Kee SJ, Kim SH, Shin MG, et al. Antifungal susceptibilities and distribution of Candida species recovered from blood cultures over an 8-year period. Korean J Lab Med 2003;329-35.
11. Shin JH, Kim HR, Lee JN. Distribution and antifungal susceptibility of Candida species isolated from clinical specimens during the past six years. Korean J Clin Microbiol 2004;164-70.
12. Lee MK, Yong D, Kim M, Kim MN, Lee K. Species distribution and antifungal susceptibilities of yeast clinical isolates from three hospitals in Korea, 2001 to 2007. Korean J Lab Med 2010;30:364-72.

13. Grassly NC, Fraser C. Seasonal infectious disease epidemiology. Proc Biol Sci 2006;273:2541-50.


14. Edi-Osagie NE, Emmerson AJ. Seasonality of invasive Candida infection in neonates. Acta Paediatr 2005;94:72-4.

15. Donders GGG, Ruban K, Donders F, Reybrouck R. Lab-based retrospective 10-year analysis shows seasonal variation of vaginal Candida infection rates in Belgium. J Clin Med 2022;11:574.


16. Kim TH, Lee JY, Chung JD, Lee SH, Lee MK. Species distribution and antifungal susceptibilities of yeast isolated from catheterized urine specimen. Korean J Urogenit Tract Infect Inflamm 2011;6:73-9.
17. Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, flucytosine, and voriconazole against Candida spp. J Clin Microbiol 2007;45:3522-8.


18. Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK 2 yeast susceptibility test with the CLSI broth microdilution reference method for testing fluconazole against Candida spp. J Clin Microbiol 2007;45:796-802.


19. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309-17.

20. Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med 2016;34:21-8.
21. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62:e1-50.


22. Hwang YY, Kang OK, Park CE, Hong SN, Kim YK, Huh HJ, et al. Frequency of Candida strains isolated from candidiasis patients at a tertiary hospital over the last 10 years. Korean J Clin Lab Sci 2022;54:110-8.
23. Tan TY, Hsu LY, Alejandria MM, Chaiwarith R, Chinniah T, Chayakulkeeree M, et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol 2016;54:471-7.

24. Astvad KMT, Johansen HK, Roder BL, Rosenvinge FS, Knudsen JD, Lemming L, et al. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 2018;56:e01564-17.


25. Ko JH, Jung DS, Lee JY, Kim HA, Ryu SY, Jung SI, et al. Changing epidemiology of non-albicans candidemia in Korea. J Infect Chemother 2019;25:388-91.

26. Kim TH, Kweon OJ, Kim HR, Lee MK. Identification of uncommon Candida species using commercial identification systems. J Microbiol Biotechnol 2016;26:2206-13.

1. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399:629-55.
2. World Health Organization. Global antimicrobial resistance surveillance system (GLASS): technical meeting on the early implementation phase. 22-23 October 2015 WHO Regional Office for Europe Copenhagen, Denmark. Meeting Report. Geneva: WHO Press; 2016 Oct. Report No.: WHO/OHE/PED/AMR/2016.1.
3. World Health Organization. GLASS early implementation protocol for inclusion of Candida spp. https://www.who.int/publications/i/item/WHO-WSI-AMR-2019.4 [Online] (last visited on 11 January 2024).
4. Lee H, Yoon EJ, Kim D, Jeong SH, Shin JH, Shin JH, et al. Establishment of the South Korean national antimicrobial resistance surveillance system, Kor-GLASS, in 2016. Euro Surveill 2018;23:1700734.
5. Korea Disease Control and Prevention Agency. National Antimicrobial Resistance Surveillance in Korea, 2021. https://www.korea.kr/docViewer/skin/doc.html?fn=197089655&rs=/docViewer/result/2022.11/17/197089655 [Online] (last visited on 11 January 2024).
6. Won EJ, Choi MJ, Jeong SH, Kim D, Shin KS, Shin JH, et al. Nationwide surveillance of antifungal resistance of Candida bloodstream isolates in South Korean hospitals: two year report from Kor-GLASS. J Fungi 2022;8:996.
7. Won EJ, Shin JH, Choi MJ, Lee WG, Park YJ, Uh Y, et al. Antifungal susceptibilities of bloodstream isolates of Candida species from nine hospitals in Korea: application of new antifungal breakpoints and relationship to antifungal usage. PLoS One 2015;10:e0118770.
8. Won EJ, Shin JH, Lee WK, Koo SH, Kim SY, Park YJ, et al. Distribution of yeast and mold species isolated from clinical specimens at 12 hospitals in Korea during 2011. Ann Clin Microbiol 2013;16:92-100.
9. Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, et al. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev 2001;14:643-58.
10. Chae MJ, Shin JH, Cho D, Kee SJ, Kim SH, Shin MG, et al. Antifungal susceptibilities and distribution of Candida species recovered from blood cultures over an 8-year period. Korean J Lab Med 2003;329-35.
11. Shin JH, Kim HR, Lee JN. Distribution and antifungal susceptibility of Candida species isolated from clinical specimens during the past six years. Korean J Clin Microbiol 2004;164-70.
12. Lee MK, Yong D, Kim M, Kim MN, Lee K. Species distribution and antifungal susceptibilities of yeast clinical isolates from three hospitals in Korea, 2001 to 2007. Korean J Lab Med 2010;30:364-72.
13. Grassly NC, Fraser C. Seasonal infectious disease epidemiology. Proc Biol Sci 2006;273:2541-50.
14. Edi-Osagie NE, Emmerson AJ. Seasonality of invasive Candida infection in neonates. Acta Paediatr 2005;94:72-4.
15. Donders GGG, Ruban K, Donders F, Reybrouck R. Lab-based retrospective 10-year analysis shows seasonal variation of vaginal Candida infection rates in Belgium. J Clin Med 2022;11:574.
16. Kim TH, Lee JY, Chung JD, Lee SH, Lee MK. Species distribution and antifungal susceptibilities of yeast isolated from catheterized urine specimen. Korean J Urogenit Tract Infect Inflamm 2011;6:73-9.
17. Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, flucytosine, and voriconazole against Candida spp. J Clin Microbiol 2007;45:3522-8.
18. Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK 2 yeast susceptibility test with the CLSI broth microdilution reference method for testing fluconazole against Candida spp. J Clin Microbiol 2007;45:796-802.
19. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309-17.
20. Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med 2016;34:21-8.
21. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62:e1-50.
22. Hwang YY, Kang OK, Park CE, Hong SN, Kim YK, Huh HJ, et al. Frequency of Candida strains isolated from candidiasis patients at a tertiary hospital over the last 10 years. Korean J Clin Lab Sci 2022;54:110-8.
23. Tan TY, Hsu LY, Alejandria MM, Chaiwarith R, Chinniah T, Chayakulkeeree M, et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol 2016;54:471-7.
24. Astvad KMT, Johansen HK, Roder BL, Rosenvinge FS, Knudsen JD, Lemming L, et al. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 2018;56:e01564-17.
25. Ko JH, Jung DS, Lee JY, Kim HA, Ryu SY, Jung SI, et al. Changing epidemiology of non-albicans candidemia in Korea. J Infect Chemother 2019;25:388-91.
26. Kim TH, Kweon OJ, Kim HR, Lee MK. Identification of uncommon Candida species using commercial identification systems. J Microbiol Biotechnol 2016;26:2206-13.