1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, 2Department of Laboratory Medicine, National Health Insurance Service Ilsan Hospital, Goyang, 3Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, 4Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, 5Department of Laboratory Medicine, Chungbuk National University College of Medicine, Cheongju, 6Department of Laboratory Medicine and Paik Institute for Clinical Research, Inje University College of Medicine, Busan, 7Department of Laboratory Medicine, Jeju National University, College of Medicine, Jeju, 8Department of Laboratory Medicine, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, 9Department of Laboratory Medicine, Yonsei University Wonju College of Medicine, Wonju, 10Department of Laboratory Medicine, Keimyung University School of Medicine, Daegu, Korea
Corresponding to Young Ah Kim, E-mail: yakim@nhimc.or.kr
Ann Clin Microbiol 2022;25(2):53-59. https://doi.org/10.5145/ACM.2022.25.2.4
Received on 25 January 2022, Revised on 1 May 2022, Accepted on 17 May 2022, Published on 20 June 2022.
Copyright © Korean Society of Clinical Microbiology.
Background: Recently, CrpP enzymes have been described as a novel cause of ciprofloxacin resistance. The crpP gene encodes a novel protein that specifically confers resistance to ciprofloxacin through an adenosine triphosphate-dependent mechanism that phosphorylates the antimicrobial. In this study, the current prevalence of the crpP gene in carbapenemaseproducing Pseudomonas aeruginosa blood isolates was evaluated.
Methods: During the study of the Antimicrobial Resistance Surveillance System in Korea, 22 blood isolates of carbapenemase-producing P. aeruginosa were collected from nine general hospitals and two nursing homes in the year 2020. Resistance genes and phylogenic trees were analyzed with the whole genome sequencing data.
Results: A total of 11 P. aeruginosa blood isolates coharbored the crpP and carbapenemase genes (nine IMP-6 producers and two GES-5-producers). Nine NDM-1-producers coharbored aac(6′)-Ib-cr and qnrVC1. One GES-9-producer also carried aac(6′)-Ib-cr, and one NDM-1producer also carried qnrVC1. The phylogenic tree showed no epidemiologic link among the 22 carbapenemase-producing P. aeruginosa isolates.
Conclusion: This is the first report on the current prevalence of the crpP gene in carbapenemaseproducing P. aeruginosa blood isolates in Korea.
Fluoroquinolone, Resistance, crpP gene, Pseudomonas aeruginosa
Pseudomonas aeruginosa, one of the antimicrobial-resistant ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter species) pathogens, represents a global threat to human health [1]. P. aeruginosa usually shows multidrug resistance. Its co-resistance to carbapenems, aminoglycosides, polymyxins, and tigecycline has also increased [2]. There are limited treatment options for serious infections caused by multidrug-resistant P. aeruginosa. Many combination therapies have been tried [3]. Recently, it has been reported that combination therapy including ciprofloxacin is correlated with a lower mortality. A combination of a beta-lactam and ciprofloxacin has been proposed for ciprofloxacin-susceptible P. aeruginosa bacteremia [4].
According to data from the Korean global antimicrobial resistance surveillance system (Kor-GLASS) [5], of P. aeruginosa blood isolates in 2017-2019, 12.3% showed resistance to piperacillin, 13.1% to ceftazidime, 12.9% to cefepime, 21.3% to imipenem, 22.1% to meropenem, 9.6% to amikacin, and 18.9% to ciprofloxacin. Most the carbapenemase-producers exhibited co-resistance to amikacin. However, data about its co-resistance to ciprofloxacin are unavailable. This study provides the newest data about the resistance mechanism to fluoroquinolone of carbapenemase-producing P. aeruginosa.
Resistance mechanisms of P. aeruginosa to fluoroquinolone are known mostly through the acquisition of mutations in genes encoding target proteins of fluoroquinolone and regulators of efflux pumps, leading to overexpression of these pumps [6]. Quinolone resistance may also be attributable to mutations of target enzymes of topoisomerases II and IV encoded by gyrA and parC, respectively [7]. These mechanisms are known to be chromosomally mediated. However, plasmid-mediated quinolone resistance (PMQR) in P. aeruginosa is also important. It is associated with qnrA-E, qnrS, qnrVC, qepA, oqxAB (efflux pump), and acc(6′)-Ib-cr (quinolone-modifying enzyme) [7]. Recently, CrpP enzymes have been described as a novel ciprofloxacin-resistance mechanism. The crpP gene encodes a novel protein, capable of specifically conferring resistance to ciprofloxacin in Escherichia coli through an adenosine triphosphate-dependent mechanism that involves phosphorylation of the antibiotic [8].
The purpose of this study was to report on the current situation of crpP gene spread in carbapenemaseproducing P. aeruginosa blood isolates in Korea.
In this study, non-duplicated P. aeruginosa blood isolates were isolated from nine nationwide general hospitals (National Health Insurance Service Ilsan Hospital, Gangnam Severance Hospital, Chonnam National University Hospital, Chungbuk National University Hospital, Busan Paik Hospital, Jeju National University Hospital, Hallym University Dongtan Sacred Heart Hospital, Wonju Severance Christian Hospital, Keimyung University Dongsan Hospital) and two nursing homes according to the Kor-GLASS manual [5] in 2020. Briefly, pure colonies of P. aeruginosa were collected in 10% skim milk and stored at -70°C before all collected isolates were transferred to a single analysis center of the Korea Disease Control and Prevention Agency forpecies were verified using analyses using approved methods [5]. Bacterial species were verified using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight mass spectrometry (Bruker Biotyper, Bruker Daltonics GmbH, Bremen, Germany). Antimicrobial susceptibility was mainly determined by the disk diffusion test according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [9].
To detect carbapenemase-producers, P. aeruginosa isolates showing nonsusceptibility to imipenem or meropenem were PCR-sequenced to detect blaKPC , blaNDM , blaOXA-48 , blaVIM , blaIMP , and blaGES . For wholegenome sequencing, DNAs of freshly subcultured isolates were extracted using a GenElute™ Bacterial Genomic DNA Kit (Sigma-Aldrich, St. Louis, MO, USA). Then 8 μg of input genomic DNA was used to sequence the entire genome using NextSeq 550 instrument (Illumina, San Diego, CA, USA). Sequences were assembled with Spades (version 3.11.1) and annotated with Prokka (version 1.13.7). Resistance genes were obtained with ResFinder 4.1 from the website of the center for genomic epidemiology [10]. A phylogenic tree was generated based on whole-genome multilocus sequence typing using a BioNumerics software, version 7.6.3 (Applied Maths, St Martens Latem, Belgium) [11] (Fig. 1).
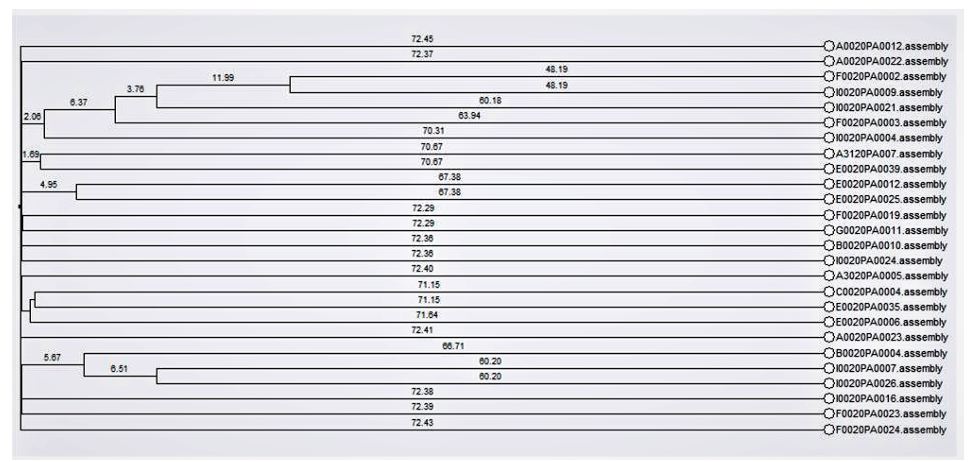
Fig. 1. Phylogenic tree of 22 carbapenemase-producing Pseudomonas aeruginosa strains based on whole genome multilocus sequence typing. Dendrogram was generated with BioNumerics software.
Among 212 non-duplicated P. aeruginosa blood isolates, 22 carbapenemase-producing P. aeruginosa isolates showed resistance to both ciprofloxacin and imipenem. A total of 11 carbapenemase-producing P. aeruginosa blood isolates (nine IMP-6 producers and two GES-5-producers) co-harbored aac(6′)-Ib-cr and crpP (Table 1). A total of nine NDM-1-producers co-harbored aac(6′)-Ib-cr and qnrVC1. One GES9-producer also carried aac(6′)-Ib-cr and one NDM-1-producer also carried qnrVC1. The phylogenic tree showed no epidemiologic link among 22 carbapenemase-producing P. aeruginosa isolates.
Table 1. Resistance to carbapenem and ciprofloxacin with corresponding resistance genes
| Isolates | Phenotype (ZD) | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|
| IPM | MEM | CIP | CRP | PMQR | gyrA | parC | ||
| F0020PA0023 | R (11) | R (10) | R (6) | blaGES-9 | aac(6′)-Ib-cr | Thr83Ile | Ser87Leu | |
| A0020PA0022 | R (7) | R (6) | R (6) | blaGES-5 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| A0020PA0005 | R (7) | R (6) | R (6) | blaGES-5 | aac(6′)-Ib-cr, crpP | Thr83Ile | WT | |
| A0020PA0023 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| A0020PA0007 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| C0020PA0004 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| E0020PA0006 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| E0020PA0012 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| E0020PA0025 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| E0020PA0035 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| E0020PA0039 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| I0020PA0016 | R (6) | R (6) | R (6) | blaIMP-6 | aac(6′)-Ib-cr, crpP | Thr83Ile | Ser87Leu | |
| B0020PA0004 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| B0020PA0010 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| F0020PA0002 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| F0020PA0003 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| I0020PA0007 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| I0020PA0009 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| I0020PA0021 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| I0020PA0024 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| I0020PA0026 | R (6) | R (6) | R (6) | blaNDM-1 | aac(6′)-Ib-cr, qnrVC1 | Thr83Ile | Ser87Leu | |
| I0020PA0004 | R (6) | R (6) | R (6) | blaNDM-1 | qnrVC1 | Thr83Ile | Ser87Leu | |
Abbreviations: IMP, imipenem; MEM, meropenem; CIP, ciprofloxacin; CRP, carbapenemase; PMQR, plasmidmediated quinolone resistance; R, resistant; ZD, zone diameter (mm); WT, wild type.
Quinolones have been commonly used as a treatment option for a large number of bacterial infections. Ciprofloxacin is the most active antibiotic in this group. The increase in quinolone resistance has limited the effect of quinolones. There was no report about the prevalence of quinolone resistance genes in P. aeruginosa in Korea to the best of our knowledge. Recently, novel mutations in gyrA and parC genes were first found in P. aeruginosa isolated from companion dogs in South Korea [12]. In that study, a total of 84 nonduplicated P. aeruginosa strains were obtained from healthy dogs and infected dogs. The resistance rate was 14.3% for levofloxacin and 13.1% for ciprofloxacin. The resistance was commonly associated with gyrA mutations [12].
The information about the prevalence of quinolone resistance genes of clinically important pathogens in Korea is limited. For K. pneumoniae, mutations in gyrA and parC were found in 78.9% and 65.5% of 142 extended-spectrum β-lactamases-producers, respectively [13]. The common PMQR gene was qnrB-aac(6′)Ib-cr-oqxAB (58/142, 40.8%) [13]. Lee et al. [14] have reported the presence of PMQR genes in Salmonella enterica isolated from human salmonellosis patients in South Korea from 2016 to 2019 [14]. Among 34 Salmonella strains with reduced susceptibility to quinolones, 25 strains harbored one or two of qnrA, qnrB, qnrS (most common), and aac(6′)-Ib-cr genes.
Out of 22 carbapenemase-producers from nationwide collections, we detected 11 P. aeruginosa blood isolates that co-harbored crpP and carbapenemase genes. This is the first report about the current situation of crpP gene spread in carbapenemase-producing P. aeruginosa blood isolates in Korea. Because all strains also have aac(6′)-Ib-cr and chromosomal mutations in gyrA or parC, the effect of the presence of crpP gene on quinolone resistance is hardly speculated in this study.
The crpP gene obtained from the pUM505 plasmid isolated from a P. aeruginosa clinical isolate was identified in 1986 [8]. Regarding the expression of CrpP in the transconjugants test, CrpP proteins increased minimal inhibitory concentration values of ciprofloxacin concerning E. coli J53-3. However, these changes were not enough to be considered as resistance according to the breakpoint value (≥ 4 mg/L) reported for this interpretation by the CLSI [8]. Nevertheless, the crpP gene conferring low-level resistance could facilitate the selection of mutants with a higher level of quinolone resistance. In addition, plasmids contain other genes in addition to the crpP gene, which encodes additional quinolone resistance mechanisms [8].
In this study, the type of carbapenemase of crpP gene-carrying P. aeruginosa was mostly IMP-6, known to be a common type in Korea [15]. NDM-1-producing P. aeruginosa isolates did not carry crpP genes, which seemed to become one of the majority types of carbapenemase in P. aeruginosa. The shift in the molecular epidemiology of carbapenemase genes might change the molecular epidemiology of PMQR genes. Mobile genetic elements play a key role in the spread of resistance genes, and high-risk clones frequently integrate such determinants into their genomes [16]. In this study, the linkage of crpP and aac(6′)-Ib-cr gene with blaIMP-6 is suspected. And there is high possibility that aac(6′)-Ib-cr and qnrVC1 are associated with blaNDM-1 in P. aeruginosa.
If only PMQR is present, it is expected to be susceptible or low-grade resistance phenotypes [7,8]. Unfortunately, the only effect of crpP gene is hard to know in this study, because all isolates had chromosomal mutations in gyrA or parC, in addition to PMQRs. These strains are usually associated with a large reduction in biological fitness with the accumulation of more resistance-associated mutations [17]. One more thing to consider is that NDM-1 carrying P. aeruginosa is mostly separated from hospitals in Daegu or Busan and the regional differences could have an effect. Therefore, we suggest continuous monitoring of the change of PMQR in P. aeruginosa.
In conclusion, the spread of P. aeruginosa isolates, coharboring crpP and carbapenemase genes were found in this study. This is the first report about the current situation of crpP gene spread in carbapenemaseproducing P. aeruginosa blood isolates in Korea.
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
Jeong Hwan Shin, Young Uh, and Nam Hee Ryoo are currently editorial board members of the Annals of Clinical Microbiology. However, they were not involved in the review process of this article.
This work was supported by a fund (2020E540600) by the Research of Korea Disease Control and Prevention Agency.
1. De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev 2020;33:e00181-19. 


2. Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection 2020;48:835-51. 


3. Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis 2019;69:S565-75. 


4. Paulsson M, Granrot A, Ahl J, Tham J, Resman F, Riesbeck K, et al. Antimicrobial combination treatment including ciprofloxacin decreased the mortality rate of Pseudomonas aeruginosa bacteraemia: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2017;36:1187-96. 


5. Kim D, Yoon EJ, Hong JS, Choi MH, Kim HS, Kim YR, et al. Major bloodstream infectioncausing bacterial pathogens and their antimicrobial resistance in South Korea, 2017-2019: phase I report from Kor-GLASS. Front Microbiol 2022;12:799084. 


6. Rehman A, Patrick WM, Lamont IL. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J Med Microbiol 2019;68:1-10. 

7. Ming DS, Chen QQ, Chen XT. Detection of 5 kinds of genes related to plasmid-mediated quinolone resistance in four species of nonfermenting bacteria with 2 drug resistant phenotypes. Can J Infect Dis Med Microbiol 2020;2020:3948719. 


8. Chávez-Jacobo VM, Hernández-Ramírez KC, Romo-Rodríguez P, Pérez-Gallardo RV, Campos-García J, Gutiérrez-Corona JF, et al. CrpP is a novel ciprofloxacin-modifying enzyme encoded by the Pseudomonas aeruginosa pUM505 plasmid. Antimicrob Agents Chemother 2018;62:e02629-17. 


9. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; CLSI Supplement M100. Wayne; PA: 2020.
10. Center for Genomic Epidemiology. Center for Genomic Epidemiology Web site. www. genomicepidemiology.org [Online] (last visited on 13 June 2022).
11. Blanc DS, Magalhães B, Koenig I, Senn L, Grandbastien B. Comparison of whole Genome (wg-) and core genome (cg-) MLST (BioNumericsTM) versus SNP variant calling for epidemiological investigation of Pseudomonas aeruginosa. Front Microbiol 2020;11:1729. 


12. Park Y, Oh J, Park S, Sum S, Song W, Chae J, et al. Antimicrobial resistance and novel mutations detected in the gyrA and parC genes of Pseudomonas aeruginosa strains isolated from companion dogs. BMC Vet Res 2020;16:111. 


13. Kim JO, Yoo IY, Yu JK, Kwon JA, Kim SY, Park YJ. Predominance and clonal spread of CTX-M-15 in cefotaxime-resistant Klebsiella pneumoniae in Korea and their association with plasmid-mediated quinolone resistance determinants. J Infect Chemother 2021;27:1186-92. 

14. Lee S, Park N, Yun S, Hur E, Song J, Lee H, et al. Presence of plasmid-mediated quinolone resistance (PMQR) genes in non-typhoidal Salmonella strains with reduced susceptibility to f luoroquinolones isolated from human salmonellosis in Gyeonggi-do, South Korea from 2016 to 2019. Gut Pathog 2021;13:35. 


15. Yoon EJ, Jeong SH. Mobile carbapenemase genes in Pseudomonas aeruginosa. Front Microbiol 2021;12:614058. 


16. Kocsis B, Toth A, Gulyas D, Ligeti B, Katona K, Rokusz L, et al. Acquired qnrVC1 and blaNDM-1 resistance markers in an international high-risk Pseudomonas aeruginosa ST773 clone. J Med Microbiol 2019;68:336-8. 

17. Lindgren PK, Marcusson LL, Sandvang D, Frimodt-Møller N, Hughes D. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob Agents Chemother 2005;49:2343-51. 


1. De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev 2020;33:e00181-19.
2. Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection 2020;48:835-51.
3. Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis 2019;69:S565-75.
4. Paulsson M, Granrot A, Ahl J, Tham J, Resman F, Riesbeck K, et al. Antimicrobial combination treatment including ciprofloxacin decreased the mortality rate of Pseudomonas aeruginosa bacteraemia: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2017;36:1187-96.
5. Kim D, Yoon EJ, Hong JS, Choi MH, Kim HS, Kim YR, et al. Major bloodstream infectioncausing bacterial pathogens and their antimicrobial resistance in South Korea, 2017-2019: phase I report from Kor-GLASS. Front Microbiol 2022;12:799084.
6. Rehman A, Patrick WM, Lamont IL. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J Med Microbiol 2019;68:1-10.
7. Ming DS, Chen QQ, Chen XT. Detection of 5 kinds of genes related to plasmid-mediated quinolone resistance in four species of nonfermenting bacteria with 2 drug resistant phenotypes. Can J Infect Dis Med Microbiol 2020;2020:3948719.
8. Chávez-Jacobo VM, Hernández-Ramírez KC, Romo-Rodríguez P, Pérez-Gallardo RV, Campos-García J, Gutiérrez-Corona JF, et al. CrpP is a novel ciprofloxacin-modifying enzyme encoded by the Pseudomonas aeruginosa pUM505 plasmid. Antimicrob Agents Chemother 2018;62:e02629-17.
9. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; CLSI Supplement M100. Wayne; PA: 2020.
10. Center for Genomic Epidemiology. Center for Genomic Epidemiology Web site. www. genomicepidemiology.org [Online] (last visited on 13 June 2022).
11. Blanc DS, Magalhães B, Koenig I, Senn L, Grandbastien B. Comparison of whole Genome (wg-) and core genome (cg-) MLST (BioNumericsTM) versus SNP variant calling for epidemiological investigation of Pseudomonas aeruginosa. Front Microbiol 2020;11:1729.
12. Park Y, Oh J, Park S, Sum S, Song W, Chae J, et al. Antimicrobial resistance and novel mutations detected in the gyrA and parC genes of Pseudomonas aeruginosa strains isolated from companion dogs. BMC Vet Res 2020;16:111.
13. Kim JO, Yoo IY, Yu JK, Kwon JA, Kim SY, Park YJ. Predominance and clonal spread of CTX-M-15 in cefotaxime-resistant Klebsiella pneumoniae in Korea and their association with plasmid-mediated quinolone resistance determinants. J Infect Chemother 2021;27:1186-92.
14. Lee S, Park N, Yun S, Hur E, Song J, Lee H, et al. Presence of plasmid-mediated quinolone resistance (PMQR) genes in non-typhoidal Salmonella strains with reduced susceptibility to f luoroquinolones isolated from human salmonellosis in Gyeonggi-do, South Korea from 2016 to 2019. Gut Pathog 2021;13:35.
15. Yoon EJ, Jeong SH. Mobile carbapenemase genes in Pseudomonas aeruginosa. Front Microbiol 2021;12:614058.
16. Kocsis B, Toth A, Gulyas D, Ligeti B, Katona K, Rokusz L, et al. Acquired qnrVC1 and blaNDM-1 resistance markers in an international high-risk Pseudomonas aeruginosa ST773 clone. J Med Microbiol 2019;68:336-8.
17. Lindgren PK, Marcusson LL, Sandvang D, Frimodt-Møller N, Hughes D. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob Agents Chemother 2005;49:2343-51.